
Postprandial hypoglycaemia in adults: pathogenesis, diagnosis and management
Introduction
Development of hypoglycaemia in a nondiabetic individual within a few hours of food ingestion is termed as postprandial hypoglycaemia (PPH). It represents a heterogeneous group of disorders characterised by inappropriate insulin secretion from the pancreatic β-cells following a meal, resulting in hypoglycaemia (Table 1).
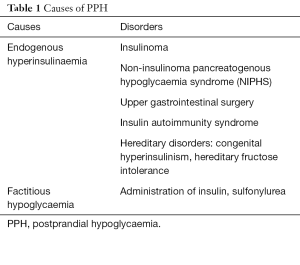
Full table
Under physiological condition, insulin secretion from the pancreatic β-cells is precisely regulated in response to changes in plasma glucose concentrations (1). A postprandial rise in blood glucose and release of glucagon-like peptide-1 (GLP-1), an incretin hormone from the small intestine, stimulate insulin synthesis and secretion from pancreatic β-cells (2). As blood glucose falls, the first defence against hypoglycaemia is a reduction in insulin secretion (3,4). When blood glucose falls below 3.8 mmol/L, there is a rapid rise in glucagon and epinephrine secretion to prevent hypoglycaemia. If hypoglycaemia continues, cortisol and growth hormone secretion increase as a delayed counter-regulatory response (3-5). Insulin secretion is completely suppressed at plasma glucose of approximately 3.0 mmol/L or lower (6-8). PPH develops when insulin secretion fails to suppress, in response to falling blood glucose concentrations and normal counter-regulatory response of glucagon and epinephrine is blunted. Excessive insulin suppresses glycogenolysis, gluconeogenesis. Therefore, hypoglycaemia results from reduced glucose production rather than its increased utilisation (4,9). Suppression of lipolysis leads to reduced ketogenesis with no alternative fuel available for cerebral metabolism. Severe hypoglycaemia with a lack of alternative fuels can cause irreversible cerebral damage. Prompt diagnosis and appropriate management of PPH is, therefore, crucial. I present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/jlpm-20-102).
Search methodology
An electronic literature search using PubMed for articles published from January 1980 till June 2020, was performed using the keywords—postprandial hypoglycaemia, reactive hypoglycaemia, hyperinsulinemic hypoglycaemia and dumping syndrome. Available publications were reviewed including their reference lists and clinically relevant articles were included.
Causes of endogenous hyperinsulinaemia
Insulinoma
Insulinoma is a rare tumour which causes endogenous hyperinsulinism and typically presents as fasting hypoglycaemia, though occasionally patients develop hypoglycaemia exclusively after meals (10). Rarely it may be associated with familial multiple endocrine neoplasia type 1 (MEN1) syndrome (11). Insulinoma will not be discussed here.
Non-insulinoma pancreatogenous hypoglycaemia syndrome (NIPHS)
This rare disorder in adults is characterised by endogenous hyperinsulinemia and diffuse nesidioblastosis. Hypoglycaemic symptoms develop within 3–4 hours after a meal while fasting blood glucose is normal (12-15). Imaging studies are negative for insulinoma, and the diagnosis is confirmed by diffuse calcium stimulated hyperinsulinemic response from multiple pancreatic vascular territories.
Its aetiology is unknown. There is no previous history of gastrointestinal tract surgery. It was first described in a group of five patients who had PPH, endogenous hyperinsulinism, normal fasting glucose, negative screening for SUR1, Kir6.2 mutation and pancreatic islet hypertrophy with nesidioblastosis (13). Diffuse nesidioblastosis has been reported in 3% of adults who underwent partial pancreatectomy to treat hyperinsulinemic hypoglycaemia (16). However, it is not clear that hyperinsulinism is solely due to the structural changes in the β-cells, as a previous study demonstrated nesidioblastosis in 36% of autopsy specimens with no history of hypoglycaemia (17).
Post-bariatric surgery hypoglycaemia
PPH is a well-known late complication of upper gastrointestinal surgery (18-22). Although it is seen more often after Roux-en-Y gastric bypass (RYGB) surgery, it may present after gastric sleeve surgery (23). It was first reported in six post-bariatric patients who underwent partial pancreatectomy to treat the symptoms. In these patients, PPH was thought to be due to nesidioblastosis, which was suggested in the pancreatic pathology (24). However, subsequent studies on the same specimens did not confirm nesidioblastosis (25).
Its exact incidence is unknown; one study reported 9.1% and 7.9% incidence at 1 and 5 years, respectively, post-RYGB surgery (20) while another study reported 2.7% and 13.3% incidence at 1 and 5 years (22). Following surgery, rapid transition of food to small intestine causes early and higher glycaemic peak and increased GLP-1 secretion, triggering larger insulin synthesis, leading to a rapid drop in blood glucose, with a nadir in 90 to 180 minutes after meals (26,27). A blunted glucagon response to insulin-induced hypoglycaemia is well documented in patients post-RYGB compared to the same patients before surgery (28). It is unclear why individual patients who develop hypoglycaemia show much enhanced pancreatic β-cell stimulation and exaggerated GLP-1 response, compared to those who do not develop hypoglycaemia after surgery (29-31). There is also reduced postprandial insulin clearance compared to asymptomatic subjects after this surgery (26,29). Recently bile acids were suggested as a metabolic regulator when postprandial unconjugated bile acids were shown to be high during hypoglycaemia in two patients with RYGB while these were normal in asymptomatic post-RYGB patients (32).
Post-bariatric hypoglycaemia mostly presents at least 6 to 12 months after surgery (10,19) and is associated with neuroglycopenic symptoms which usually appear 1 to 3 hours after meals. Plasma glucose is <2.7 mmol/L and symptoms are relieved with improved blood glucose concentration (10,19). There is no fasting hypoglycaemia. Many patients may develop symptoms of dumping syndrome in the first 6 months of post-bariatric period related to adrenergic stimulation such as perspiration, palpitations, tremor and irritability (33-35).
Insulin autoimmunity
In this extremely rare condition, insulin antibodies or anti-insulin receptor antibodies develop and patient presents with hypoglycaemia which may be in late postprandial state (42%), fasting state (31%) or both (24%) (36-40). Plasma insulin levels are typically high, and antibodies are detected in the plasma. This disorder is discussed in detail in another review.
Congenital endogenous hyperinsulinemia
This disorder is due to dysregulated insulin secretion, secondary to a mutation in specific genes (ABCC8, KCNJ11, GLUD1, HADH) which regulate insulin secretion from pancreatic β-cells. It is mainly seen in infants and children (41,42). However, a few milder late-onset forms have been reported in adults (43,44). Patients usually show sensitivity to specific proteins in the meal and develop postprandial hyperinsulinemic hypoglycaemia (42-44). A mutation in INSR (insulin receptor kinase) gene leading to impaired insulin clearance and insulin resistance, results in PPH with onset in adolescence to adulthood (45).
Hereditary fructose intolerance
This disorder develops due to aldolase B deficiency. It manifests as PPH following fructose or sucrose ingestion, which leads to accumulation of fructose-1-phosphate, inhibiting glycogenolysis and gluconeogenesis. Several cases have been diagnosed late in adulthood (46,47).
Factitious hypoglycaemia (exogenous hyperinsulinemia)
Exogenous hyperinsulinemia causing PPH, due to surreptitious administration of insulin or insulin secretagogues such as sulfonylureas and meglitinides, is usually seen in people who have access to these medications.
Hypoglycaemia symptoms
Symptoms of hypoglycaemia are categorised as autonomic and neuroglycopenic symptoms. Autonomic symptoms such as tremors, anxiety, palpitation, sweating are due to adrenergic and partly cholinergic stimulation, triggered by hypoglycaemia and develop at a plasma glucose concentration of approximately 3.3 mmol/L (48,49). Perception of these symptoms provides awareness of hypoglycaemia (49). Neuroglycopenic symptoms such as dizziness, light headedness, blurred vision, diplopia, cognitive impairment, behavioural changes, slurred speech, seizures and coma are typically seen in PPH. These are due to cerebral glucose deprivation, and develop at plasma glucose levels of approximately 2.7 mmol/L and lower (6,49). Patients may experience symptoms at different glycaemic thresholds, and with recurrent episodes of hypoglycaemia, they may lose hypoglycaemic awareness (10).
Patient evaluation
These patients are mostly healthy-looking individuals who present with nonspecific symptoms suggestive of hypoglycaemia, after a meal. It is crucial to confirm the presence of hypoglycaemia associated with symptoms or signs that are relieved after raising blood glucose concentration (Whipple’s triad) (10,50). This may be challenging as the patient may not be able to go to the lab during symptoms, and the use of glucometer during symptoms may give false reading due to inadequate technique, and the method may not give accurate result at low glucose concentrations (10,51). Hypoglycaemia is confirmed by detecting blood glucose <2.7 mmol/L and plasma glucose <3.0 mmol/L measured from a venous blood sample with a precise laboratory method. Preanalytical errors such as a collection of the blood sample in a tube without a glycolysis inhibitor (sodium fluoride or ethylenediaminetetraacetic acid-citrate: EDTA) and delayed separation of the plasma from the formed elements in blood, may result in continued glucose metabolism giving false low glucose concentration- pseudo-hypoglycaemia particularly in the presence of erythrocytosis, leucocytosis, or thrombocytosis (10). Postprandial hypoglycaemic symptoms without Whipple’s triad, previously termed as “reactive hypoglycaemia” is a functional disorder now known as a postprandial syndrome, in which symptoms are not due to hypoglycaemia (52).
Once Whipple’s triad is established, a comprehensive medical history should include the details of symptoms; timing in relation to meals; duration; provoking and alleviating factors; any prescribed or over the counter drug intake; any surgery on upper gastrointestinal tract including bariatric surgery; any known critical illness or hormonal deficiency and any similar family history. These patients’ physical examination is usually unremarkable with no evidence of critical illness, hormone deficiency, or non-islet cell tumour.
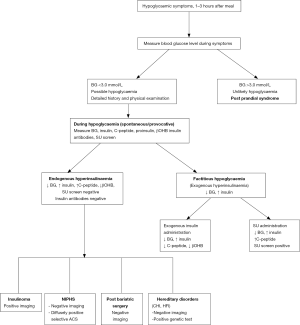
Further evaluation of hypoglycaemia should be carried out during an episode of spontaneous hypoglycaemia when blood samples are collected to measure glucose, insulin, C-peptide, proinsulin, β-hydroxybutyrate (β-OHB), insulin antibodies and for oral hypoglycaemic agents screen, to confirm different causes of PPH (Figure 1). Diagnosis of endogenous hyperinsulinemia is confirmed when in the presence of plasma glucose of <3.0 mmol/L, there is detectable plasma insulin (3 µU/mL; 18 pmol/L) with high C-peptide (0.2 nmol/L), proinsulin of 5.0 pmol/L, suppressed plasma free fatty acid (<1.5 mmol/L) and β-OHB (≤2.7 mmol/L) (8,10,53).
Provocative test
If further investigation is not possible during a spontaneous episode of hypoglycaemia, a mixed meal test is recommended to recreate PPH settings (10). Oral glucose tolerance test has no role in the evaluation of PPH (10,54). A mixed-meal test should be performed under the supervision of trained personnel who can promptly intervene to manage severe hypoglycaemia. After an overnight fast, the patient is given a meal containing protein, carbohydrates, and fat that provokes symptoms. Blood samples are taken for baseline plasma glucose, insulin, C peptide, proinsulin, β-OHB, and then every 30 minutes for 5 hours. Fasting blood glucose is typically normal in these patients except in those with insulinoma. Neuroglycopenic symptoms develop at a blood glucose concentration of <2.7 mmol/L and plasma glucose <3.0 mmol/L. Blood samples are also taken for measuring any circulating oral hypoglycaemic agents (sulfonylureas, glinides) during hypoglycaemia and insulin antibodies to differentiate between various causes of PPH (Figure 1).
Imaging
Imaging studies such as abdominal computed tomography, MRI, and endoscopic pancreatic ultrasonography with fine-needle aspiration are required to detect insulinoma as a cause of PPH (55,56). Selective pancreatic arterial calcium stimulation test, confirms the diagnosis of NIPHS by demonstrating diffuse calcium-stimulated hyperinsulinism from multiple segments of pancreatic vascular supply. It also helps differentiate NIPHS from a solitary tiny insulinoma that is not apparent on standard imaging procedures (56,57). New imaging modalities like fluorine-18-L-dihydroxyphenylalanine positron emission tomography (18F-DOPA PET) and PET/CT with 68Ga-DOTA-exendin-4 are helpful in precisely localising insulinoma (58-60).
Management of PPH
General measures
Patient education is crucial in managing hypoglycaemia as they are at risk of accidents and injuries due to neuroglycopenic symptoms. They should learn to monitor their capillary blood glucose by a glucose meter, when symptomatic. Family members should be educated to be aware of hypoglycaemic symptoms and the use of glucagon injection in an emergency.
Emergency management
Severe symptoms are treated with glucose tablet/gel or intramuscular glucagon injection, which raises blood glucose concentration by inducing glycogenolysis and gluconeogenesis. Those who require attendance at the hospital with loss of consciousness and seizures are treated with 50% dextrose or glucagon followed by dextrose infusion.
Long term management
Dietary modifications
This is the first step in the management of PPH. The patient is advised to maintain a food diary to identify provocative food. A diet with low carbohydrate, high protein content with added fibres and fat helps reduce post-meal glycaemic excursions and ameliorate hypoglycaemia (61,62). Use of complex carbohydrate with a low glycaemic index is advised while simple carbohydrate with high glycaemic index should be avoided (62). Uncooked corn starch was used in a study for preventing symptoms hyperinsulinaemic hypoglycaemia of infancy (63); however, it may not work in all cases.
Acarbose
It is an α-glucosidase inhibitor, which delays the intestinal breakdown of complex carbohydrate to glucose, reducing postprandial glucose and insulin surge and subsequent hypoglycaemia (64,65). Although it is the first-line medication for postprandial hyperinsulinemic hypoglycaemia, its use is limited by the side effects like flatulence and diarrhoea.
Diazoxide
It inhibits insulin secretion by inhibition of β-cell ATP-sensitive potassium channels (66). It is used for controlling hyperinsulinemic symptoms in insulinoma and other causes of endogenous hyperinsulinaemic hypoglycaemia.
Somatostatin analogues
Octreotide is used when a dietary modification, acarbose and diazoxide are ineffective. It binds to somatostatin receptor-2 and receptor-5 located on β-pancreatic cells, inhibiting calcium influx and reducing insulin synthesis and secretion (67). Its long-acting preparation, lanreotide, which is given once monthly, may show better results in some patients. However, it loses its efficacy over time because of gradual internalisation of somatostatin receptors, when the patient becomes desensitised to the medication. A combination of somatostatin analogue and diazoxide may prove helpful in some patients (68). Side-effects of octreotide include diarrhoea, risks of cholelithiasis and prolonged QT interval.
Novel therapies
GLP-1 receptor antagonist, exendin-9, has been reported to be effective in preventing PPH and maintaining plasma glucose stability (27,69). Sirolimus, an immunosuppressant, has been successfully used in children with severe hyperinsulinaemic hypoglycaemia, unresponsive to maximum doses of diazoxide and octreotide (70).
Surgery
Surgical resection of insulinoma is the preferred treatment. Nevertheless, surgery is the last option to treat other causes of severe endogenous hyperinsulinism, refractory to dietary modification and medical therapy. A gradient graded or distal pancreatectomy is the treatment of choice in severely affected refractory cases of NIPHS (14,15). Partial pancreatectomy has not been successful in post-bariatric cases, as most patients continue to have symptoms (71). Feeding through gastrostomy tube placed into the remnant stomach has been effectively used in some post-RYGB cases, as the nutrients pass through the duodenum and upper jejunum with normal glucose, insulin and GLP1 response (72,73). Reversal of RYGB may relieve the refractory hypoglycaemic symptoms in cases, unresponsive to other measures (74,75).
Conclusions
PPH in adults is not common; however, with an increasing number of bariatric procedures worldwide, clinicians are encountering more patients with this complication. Its evaluation and management can be arduous and challenging. Further studies are required to explore novel therapies to prevent and treat neuroglycopenia that not only increases morbidity, reduces the quality of life, and it also impairs patient safety as they are more prone to have accidents.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Rousseau Gama) for the series “Adult Spontaneous Hypoglycaemia” published in Journal of Laboratory and Precision Medicine. The article has undergone external peer review.
Reporting Checklist: The author has completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/jlpm-20-102
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm-20-102). The series “Adult Spontaneous Hypoglycaemia” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rorsman P, Braun M. Regulation of insulin secretion in human pancreatic islets. Annu Rev Physiol 2013;75:155-79. [Crossref] [PubMed]
- Jones B, Bloom SR, Buenaventura T, et al. Control of insulin secretion by GLP-1. Peptides 2018;100:75-84. [Crossref] [PubMed]
- Cryer PE, Gerich JE. Glucose counter-regulation, hypoglycemia, and intensive insulin therapy in diabetes mellitus. N Engl J Med 1985;313:232-41. [Crossref] [PubMed]
- Cryer PE, White NH, Santiago JV. The relevance of glucose counter regulatory systems to patients with insulin-dependent diabetes mellitus. Endocr Rev 1986;7:131-9. [Crossref] [PubMed]
- Cryer PE. Hypoglycemia, functional brain failure, and brain death. J Clin Invest 2007;117:868-70. [Crossref] [PubMed]
- Mitrakou A, Ryan C, Veneman T, et al. Hierarchy of glycemic thresholds for counterregulatory hormone secretion, symptoms, and cerebral dysfunction. Am J Physiol 1991;260:E67-74. [PubMed]
- Heller SR, Cryer PE. Hypoinsulinemia is not critical to glucose recovery from hypoglycemia in humans. Am J Physiol 1991;261:E41-8. [PubMed]
- Service FJ. Hypoglycemic disorders. N Engl J Med 1995;332:1144-52. [Crossref] [PubMed]
- Rizza RA, Haymond MW, Verdonk CA, et al. Pathogenesis of hypoglycemia in insulinoma patients: suppression of hepatic glucose production by insulin. Diabetes 1981;30:377-81. [Crossref] [PubMed]
- Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and management of adult hypoglycemic disorders: Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2009;94:709-28. [Crossref] [PubMed]
- Service FJ, McMahon MM, O’Brien PC, et al. Functioning insulinoma—incidence, recurrence, and long-term survival of patients: a 60-year study. Mayo Clin Proc 1991;66:711-9. [Crossref] [PubMed]
- Anlauf M, Wieban D, Perren A, et al. Persistent hyperinsulinemic hypoglycemia in 15 adults with diffuse nesidioblastosis: diagnostic criteria, incidence, characterisation of beta-cell changes. Am J Surg Pathol 2005;29:524-33. [Crossref] [PubMed]
- Service FJ, Natt N, Thompson GB, et al. Noninsulinoma pancreatogenous hypoglycemia: a novel syndrome of hyperinsulinemic hypoglycemia in adults independent of mutations in Kir6.2 and SUR1 genes. J Clin Endocrinol Metab 1999;84:1582-9. [Crossref] [PubMed]
- Thompson GB, Service FJ, Andrews JC, et al. Noninsulinoma pancreatogenous hypoglycemia syndrome: an update in 10 surgically treated patients. Surgery 2000;128:937-44; discussion 944-5. [Crossref] [PubMed]
- Won JG, Tseng HS, Yang AH, et al. Clinical features and morphological characterisation of 10 patients with noninsulinoma pancreatogenous hypoglycaemia syndrome (NIPHS). Clin Endocrinol (Oxf) 2006;65:566-78. [Crossref] [PubMed]
- Raffel A, Krausch MM, Anlauf M, et al. Diffuse nesidioblastosis as a cause of hyperinsulinemic hypoglycemia in adults: a diagnostic and therapeutic challenge. Surgery 2007;141:179-84. [Crossref] [PubMed]
- Karnauchow PN. Nesidioblastosis in adults without insular hyperfunction. Am J Clin Pathol 1982;78:511-3. [Crossref] [PubMed]
- Marsk R, Jonas E, Rasmussen F, et al. Nationwide cohort study of post-gastric bypass hypoglycaemia including 5040 patients undergoing surgery for obesity in 1986–2006 in Sweden. Diabetologia 2010;53:2307-11. [Crossref] [PubMed]
- Salehi M, Vella A, McLaughlin T, et al. Hypoglycemia after gastric bypass surgery: current concepts and controversies. J Clin Endocrinol Metab 2018;103:2815-26. [Crossref] [PubMed]
- Raverdy V, Baud G, Pigeyre M, et al. Incidence and predictive factors of postprandial hyperinsulinemic hypoglycemia After Roux-en-Y gastric bypass: a five year longitudinal study. Ann Surg 2016;264:878-85. [Crossref] [PubMed]
- Lee CJ, Clark JM, Schweitzer M, et al. Prevalence of and risk factors for hypoglycemic symptoms after gastric bypass and sleeve gastrectomy. Obesity (Silver Spring) 2015;23:1079-84. [Crossref] [PubMed]
- Lee CJ, Wood GC, Lazo M, et al. Risk of post-gastric bypass surgery hypoglycemia in nondiabetic individuals: a single center experience. Obesity (Silver Spring) 2016;24:1342-8. [Crossref] [PubMed]
- Belligoli A, Sanna M, Serra R, et al. Incidence and predictors of hypoglycemia 1 year after laparoscopic sleeve gastrectomy. Obes Surg 2017;27:3179-86. [Crossref] [PubMed]
- Service GJ, Thompson GB, Service FJ, et al. Hyperinsulinemic Hypoglycemia with Nesidioblastosis after Gastric-Bypass Surgery. N Engl J Med 2005;353:249-54. [Crossref] [PubMed]
- Meier JJ, Butler AE, Galasso R, et al. Hyperinsulinemic hypoglycemia after gastric bypass surgery is not accompanied by islet hyperplasia or increased beta-cell turnover. Diabetes Care 2006;29:1554-9. [Crossref] [PubMed]
- Salehi M, Gastaldelli A, D'Alessio DA. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. J Clin Endocrinol Metab 2014;99:2008-17. [Crossref] [PubMed]
- Salehi M, Gastaldelli A, D'Alessio DA. Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology 2014;146:669-80.e2. [Crossref] [PubMed]
- Abrahamsson N, Börjesson JL, Sundbom M, et al. Gastric bypass reduces symptoms and hormonal responses in hypoglycemia. Diabetes 2016;65:2667-75. [Crossref] [PubMed]
- Toft-Nielsen M, Madsbad S, Holst JJ. Exaggerated secretion of glucagon-like peptide-1 (GLP-1) could cause reactive hypoglycaemia. Diabetologia 1998;41:1180-6. [Crossref] [PubMed]
- Honka H, Salehi M. Postprandial hypoglycemia after gastric bypass surgery: from pathogenesis to diagnosis and treatment. Curr Opin Clin Nutr Metab Care 2019;22:295-302. [Crossref] [PubMed]
- Foster-Schubert KE. Hypoglycemia complicating bariatric surgery: incidence and mechanisms. Curr Opin Endocrinol Diabetes Obes 2011;18:129-33. [Crossref] [PubMed]
- Honka H, D’Alessio DA, DeFronzo RA, et al. Altered bile acid metabolism after gastric bypass in subjects with and without hypoglycemia. Obesity Week 2018:abstr T-P-3266.
- Tack J, Arts J, Caenepeel P, et al. Pathophysiology, diagnosis and management of postoperative dumping syndrome. Nat Rev Gastroenterol Hepatol 2009;6:583-90. [Crossref] [PubMed]
- Tack J, Deloose E. Complications of bariatric surgery: dumping syndrome, reflux and vitamin deficiencies. Best Pract Res Clin Gastroenterol 2014;28:741-9. [Crossref] [PubMed]
- Emous M, Wolffenbuttel BHR, Totté E, et al. The short- to mid-term symptom prevalence of dumping syndrome after primary gastric-bypass surgery and its impact on health-related quality of life. Surg Obes Relat Dis 2017;13:1489-500. [Crossref] [PubMed]
- Goldman J, Baldwin D, Rubenstein AH, et al. Characterisation of circulating insulin and proinsulin-binding antibodies in autoimmune hypoglycemia. J Clin Invest 1979;63:1050-9. [Crossref] [PubMed]
- Hirata Y, Ishizu H. Elevated insulin-binding capacity of serum proteins in a case with spontaneous hypoglycemia and mild diabetes not treated with insulin. Tohoku J Exp Med 1972;107:277-86. [Crossref] [PubMed]
- Lupsa BC, Chong AY, Cochran EK, et al. Autoimmune forms of hypoglycemia. Medicine (Baltimore) 2009;88:141-53. [Crossref] [PubMed]
- Redmon JB, Nuttall FQ. Autoimmune hypoglycemia. Endocrinol Metab Clin North Am 1999;28:603-18. [Crossref] [PubMed]
- Arioglu E, Anderwelt A, Diabo C, et al. Clinical course of the syndrome of autoantibodies to the insulin receptor (type B insulin resistance): a 28-year perspective. Medicine (Baltimore) 2002;81:87-100. [Crossref] [PubMed]
- Senniappan S, Shanti B, James C, et al. Hyperinsulinaemic hypoglycaemia: genetic mechanisms, diagnosis and management. J Inherit Metab Dis 2012;35:589-601. [Crossref] [PubMed]
- Shah P, Rahman SA, Demirbilek H, et al. Hyperinsulinaemic hypoglycaemia in children and adults. Lancet Diabetes Endocrinol 2017;5:729-42. [Crossref] [PubMed]
- Babiker O, Flanagan SE, Ellard S, et al. Protein-induced hyperinsulinaemic hypoglycaemia due to a homozygous HADH mutation in three siblings of a Saudi family. J Pediatr Endocrinol Metab 2015;28:1073-7. [Crossref] [PubMed]
- Gutgold A, Gross DJ, Glaser B, et al. Diagnosis of ABCC8 congenital hyperinsulinism of infancy in a 20-year-old man evaluated for factitious hypoglycemia. J Clin Endocrinol Metab 2017;102:345-9. [PubMed]
- Højlund K, Hansen T, Lajer M, et al. A novel syndrome of autosomal-dominant hyperinsulinemic hypoglycemia linked to a mutation in the human insulin receptor gene. Diabetes 2004;53:1592-8. [Crossref] [PubMed]
- Yasawy MI, Folsch UR, Schmidt WE, et al. Adult hereditary fructose intolerance. World J Gastroenterol 2009;15:2412-3. [Crossref] [PubMed]
- Lameire N, Mussche M, Baele G, et al. Hereditary fructose intolerance: a difficult diagnosis in the adult. Am J Med 1978;65:416-23. [Crossref] [PubMed]
- Hepburn DA, Deary IJ, Frier BM, et al. Symptoms of acute insulin-induced hypoglycemia in humans with and without IDDM. Factor-analysis approach. Diabetes Care 1991;14:949-57. [Crossref] [PubMed]
- Towler DA, Havlin CE, Craft S, et al. Mechanism of awareness of hypoglycemia. Perception of neurogenic (predominantly cholinergic) rather than neuroglycopenic symptoms. Diabetes 1993;42:1791-8. [Crossref] [PubMed]
- Whipple AO. The surgical therapy of hyperinsulinism. J Int Chir 1938;3:237-76.
- American Diabetes Association. Consensus statement on self-monitoring of blood glucose. Diabetes Care 1987;10:95-9. [Crossref] [PubMed]
- Palardy J, Havrankova J, Lepage R, et al. Blood glucose measurements during symptomatic episodes in patients with suspected postprandial hypoglycemia. N Engl J Med 1989;321:1421-5. [Crossref] [PubMed]
- Guettier JM, Gorden P. Hypoglycemia. Endocrinol Metab Clin North Am 2006;35:753-66. viii-ix. [Crossref] [PubMed]
- Gama R, Teale JD, Marks V. Clinical and laboratory investigation of adult spontaneous hypoglycaemia. J Clin Pathol 2003;56:641-6. [Crossref] [PubMed]
- Noone TC, Hosey J, Firat Z, et al. Imaging and localization of islet-cell tumours of the pancreas on CT and MRI. Best Pract Res Clin Endocrinol Metab 2005;19:195-211. [Crossref] [PubMed]
- Grant CS. Insulinoma. Best Pract Res Clin Gastroenterol 2005;19:783-98. [Crossref] [PubMed]
- Wiesli P, Brändle M, Schmid C, et al. Selective arterial calcium stimulation and hepatic venous sampling in the evaluation of hyperinsulinemic hypoglycemia: potential and limitations. J Vasc Interv Radiol 2004;15:1251-6. [Crossref] [PubMed]
- Christiansen CD, Petersen H, Nielsen AL, et al. 18F-DOPA PET/CT and 68Ga-DOTANOC PET/CT scans as diagnostic tools in focal congenital hyperinsulinism: a blinded evaluation. Eur J Nucl Med Mol Imaging 2018;45:250-61. [Crossref] [PubMed]
- Kauhanen S, Seppänen M, Minn H, et al. Fluorine-18-L-dihydroxyphenylalanine (18F-DOPA) positron emission tomography as a tool to localize an insulinoma or beta-cell hyperplasia in adult patients. J Clin Endocrinol Metab 2007;92:1237-44. [Crossref] [PubMed]
- Cuthbertson DJ, Banks M, Khoo B, et al. Application of Ga(68)-DOTA-exendin-4 PET/ CT to localise an occult insulinoma. Clin Endocrinol (Oxf) 2016;84:789-91. [Crossref] [PubMed]
- Kellogg TA, Bantle JP, Leslie DB, et al. Postgastric bypass hyperinsulinemic hypoglycemia syndrome: characterisation and response to a modified diet. Surg Obes Relat Dis 2008;4:492-9. [Crossref] [PubMed]
- Suhl E, Anderson-Haynesa SE, Mulla C, et al. Medical nutrition therapy for post-bariatric hypoglycemia: practical insights. Surg Obes Relat Dis 2017;13:888-96. [Crossref] [PubMed]
- Suprasongsin C, Suthutvoravut U, Mahachoklertwattana P, et al. Combined raw cornstarch and nifedipine as an additional treatment in persistent hyperinsulinemic hypoglycemia of infancy. J Med Assoc Thai 1999;82:S39-42. [PubMed]
- Ritz P, Vaurs C, Bertrand M, et al. Usefulness of acarbose and dietary modifications to limit glycemic variability following Roux-en-Y gastric bypass as assessed by continuous glucose monitoring. Diabetes Technol Ther 2012;14:736-40. [Crossref] [PubMed]
- Valderas JP, Ahuad J, Rubio L, et al. Acarbose improves hypoglycaemia following gastric bypass surgery without increasing glucagon-like peptide 1 levels. Obes Surg. 2012;22:582-6. [Crossref] [PubMed]
- Roženková K, Güemes M, Shah P, et al. The diagnosis and management of hyperinsulinaemic hypoglycaemia. J Clin Res Pediatr Endocrinol 2015;7:86-97. [Crossref] [PubMed]
- Welters A, Lerch C, Kummer S, et al. Long-term medical treatment in congenital hyperinsulinism: a descriptive analysis in a large cohort of patients from different clinical centers. Orphanet J Rare Dis 2015;10:150. [Crossref] [PubMed]
- Mohammadi A, Sulaiman RA, Grossman AB. Pasireotide and octreotide in the treatment of severe late dumping syndrome. Clin Case Rep 2017;5:1608-11. [Crossref] [PubMed]
- Kostopoulou E, Shah P. Hyperinsulinaemic hypoglycaemia-an overview of a complex clinical condition. Eur J Pediatr 2019;178:1151-60. [Crossref] [PubMed]
- Senniappan S, Alexandrescu S, Tatevian N, et al. Sirolimus therapy in infants with severe hyperinsulinemic hypoglycemia. N Engl J Med 2014;370:1131-7. [Crossref] [PubMed]
- Vanderveen KA, Grant CS, Thompson GB, et al. Outcomes and quality of life after partial pancreatectomy for noninsulinoma pancreatogenous hypoglycemia from diffuse islet cell disease. Surgery 2010;148:1237-45. [Crossref] [PubMed]
- McLaughlin T, Peck M, Holst J, et al. Reversible hyperinsulinemic hypoglycemia after gastric bypass: a consequence of altered nutrient delivery. J Clin Endocrinol Metab 2010;95:1851-5. [Crossref] [PubMed]
- Craig CM, Lamendola C, Holst JJ, et al. The use of gastrostomy tube for the long-term remission of hyperinsulinemic hypoglycemia after Roux-en-y gastric bypass: a case report. AACE Clin Case Rep 2015;1:e84-7. [Crossref]
- Campos GM, Ziemelis M, Paparodis R, et al. Laparoscopic reversal of Roux-en-Y gastric bypass: technique and utility for treatment of endocrine complications. Surg Obes Relat Dis 2014;10:36-43. [Crossref] [PubMed]
- Shoar S, Nguyen T, Ona MA, et al. Roux-en-Y gastric bypass reversal: a systematic review. Surg Obes Relat Dis 2016;12:1366-72. [Crossref] [PubMed]
Cite this article as: Sulaiman RA. Postprandial hypoglycaemia in adults: pathogenesis, diagnosis and management. J Lab Precis Med 2021;6:13.


