
Inherited metabolic disorders associated with hypoglycaemia in adulthood: a narrative review
Introduction
Inherited metabolic disorders (IMD) are conditions caused by mutations in a gene encoding an enzyme, transporter protein or enzyme cofactor involved in generating energy or removing waste products of metabolism. Glucose is an essential source of energy for brain development and function and developing paediatric brains are especially susceptible to the consequences of hypoglycaemia. Therefore, many of the IMDs associated with hypoglycaemia have historically been better understood as disorders of childhood. They are well described in textbooks but often with a paediatric focus and grouped according to the metabolic defect rather than the symptoms they cause. A clinician presented with an adult patient with hypoglycaemia may therefore find many traditional textbooks of limited value for formulating a differential diagnosis, planning investigations or for furthering their understanding of how the conditions affect adults. In this article this gap in the literature is addressed by coming from a starting point of the adult presenting with hypoglycaemia. A clinical and diagnostic algorithm is proposed followed by a more detailed description of the disorders making up the differential diagnosis. Only IMDs in which hypoglycaemia persists into adulthood are considered, it is beyond the scope of this article to consider all of the IMD causes of paediatric hypoglycaemia. The article is primarily written for educational purposes and does not attempt to offer new insights into these well-documented conditions. We present the article in accordance with the narrative review reporting checklist provided (available at http://dx.doi.org/10.21037/jlpm-20-100).
Methods
Information used to write this article was based on the author’s clinical experience of standard operating procedures for diagnosing and managing adults with hypoglycaemia and IMDs, personal libraries of texts on IMDs and hand searches of references of retrieved literature.
Discussion
The central role of glucose as a source of energy means that glucose homeostasis is critical for health. In a healthy individual a constant blood glucose concentration is maintained by the pancreatic hormones, insulin, glucagon, and somatostatin interacting in negative feedback loops on the metabolic pathways of glycolysis, glycogenolysis and gluconeogenesis. Glucose is produced from the metabolism of stored glycogen via glycogenolysis and generated by gluconeogenesis either from dietary fructose or by de novo synthesis from non-carbohydrate sources (alanine and lactate) via pyruvate. Glycolysis is the breakdown of glucose to generate energy in the mitochondrion via the Krebs cycle. Pyruvate lies at a critical juncture between energy generation via glycolysis and glucose synthesis via gluconeogenesis. Pyruvate is metabolised irreversibly either to acetyl CoA for energy generation in the Krebs cycle or to the Krebs cycle intermediate, oxaloacetate which is converted to phosphoenolpyruvate to re-enter the gluconeogenesis pathway. Fatty acid metabolism is the other major source of acetyl CoA for energy generation or gluconeogenesis via the Krebs cycle (Figure 1).
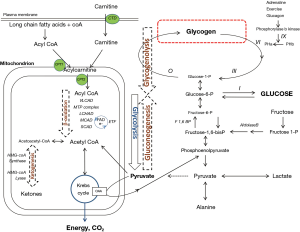
IMDs associated with hypoglycaemia are caused by pathogenic mutations in genes encoding any one of the enzymes or transport proteins involved in glycogenolysis, gluconeogenesis or fatty acid metabolism.
With a few exceptions, IMDs affecting glucose production are autosomal recessive. This means that to be clinically affected an individual must possess two pathogenic mutations (one from each parent) on separate chromosomes of the same gene. For most IMDs there is a spectrum of disease severity that, to some extent, can be predicted by knowledge of the biochemical effect of the causative mutations. Mutations that allow for some residual enzyme activity, including those caused by compound heterozygosity for one severe and one milder mutation, cause attenuated phenotypes manifesting as milder and/or later onset disease compared with those which completely nullify translation of functional enzyme.
In children presenting with spontaneous hypoglycaemia IMDs routinely form part of the differential diagnosis. However, as the nervous system matures, the frequency and severity of hypoglycaemic episodes tends to wane. Thus, in adults with attenuated phenotypes, other features of these conditions generally predominate. Huge advances in gene sequencing technologies in recent years and better lifetime care has advanced our understanding of these attenuated phenotypes and how IMDs affect adults.
Diagnosis of IMDs causing hypoglycaemia
In adulthood, true pathogenic hypoglycaemia is defined as a documented blood glucose concentration of less than 3 mmol/L (less than 0.55 g/L). Many individuals subjectively report hypoglycaemic symptoms at higher blood glucose concentrations and therefore it is essential that true hypoglycaemia is established prior to further investigation.
Hypoglycaemia stimulates the autonomic nervous system to produce adrenergic (tremor, tachycardia, hypertension) and cholinergic (sweat, hunger) responses. If uncorrected, a low central nervous system glucose level precipitates encephalopathy (drowsiness, confusion, seizures). Presence of the Whipple’s triad of true hypoglycaemia and hypoglycaemic symptoms which resolve when the glucose level is raised should always prompt further investigation (1). However absence of these features does not preclude an IMD diagnosis. Patients with frequent hypoglycaemic episodes associated with a longstanding poorly managed (or previously undiagnosed) IMD can lack hypoglycaemic awareness.
It is essential to note that true hypoglycaemia presenting for the first time in adulthood is significantly more likely to be caused by an endocrine disorder than an IMD. A diagnostic algorithm for endocrine disorders causing hypoglycaemia is detailed in the Endocrine Society clinical practice guideline (1). Initial laboratory tests are as follows:
- Routine biochemistry (renal function, liver function, creatine kinase, C-reactive protein, lipid profile, urate);
- Endocrine tests (insulin, c-peptide, cortisol, growth hormone, insulin-like growth factor 1, oral hypoglycaemic agents)—these samples should ideally be collected during the hypoglycaemic episode.
After exclusion of endocrine disorders, a helpful clue to direct further investigations comes from determining whether hypoglycaemia occurs in the fasting or post-prandial state.
Subsequent screening investigations oriented towards an IMD diagnosis include, lactate, acylcarnitines and free carnitine, free fatty acids (FFA) and 3-βhydroxybutyrate (3-OHB), urine dipstick for ketones, and urine organic acids. These are described in more detail in the sections to follow and listed in Table 1 alongside the IMD with which abnormal results are most associated.
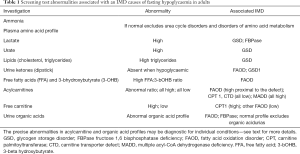
Full table
In addition to the screening investigations listed above, measurement of quantitative plasma amino acids and ammonia are indicated to exclude IMDs causing hyperammonaemic rather than hypoglycaemic encephalopathy. These include disorders of amino acid metabolism, organic acidurias and urea cycle disorders and will not be discussed further in this article.
IMD causes of fasting hypoglycaemia are sub-divided into those associated with ketone production and those in which ketones are absent. In the fasting state, up to 80% of the total energy requirement is normally met by ketones generated from the metabolism of FFA. Thus, the absence of ketones in a fasted individual is a key diagnostic clue pointing towards a fatty acid oxidation disorder or glycogen storage disorder type I (GSD I).
Conversely, ketotic hypoglycaemia has a wide potential differential diagnosis including non-IMD causes which must be excluded first. IMD causes are other GSDs and fructose 1,6 bisphosphatase deficiency, a disorder of gluconeogenesis.
Post-prandial hypoglycaemia is a relatively common complaint in adulthood and, similarly, non-IMD causes must be excluded first. It is also a feature of hereditary fructose intolerance (HFI) and three of the congenital disorders of glycosylation (CDG). An algorithm for recalling the IMD causes of fasting and post-prandial hypoglycaemia is shown in Figure 2.
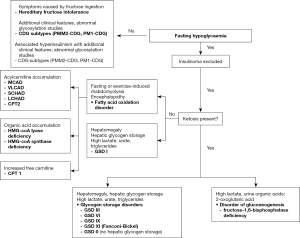
For a comprehensive review of the metabolic pathways and the defects that give rise to the IMDs discussed in this article the reader is encouraged to refer to the relevant chapters in the textbook, Inborn Metabolic Diseases edited by Saudubray et al. (2).
IMDs causing fasting hypoglycaemia in adulthood
Fatty acid oxidation disorders
A major source of cellular energy is derived from mitochondrial fatty acid oxidation. At times of increased catabolism, for example during fasting or prolonged exercise, long chain triglycerides stored in body fat are released by lipases and activated to acyl-coA esters which are transported into the mitochondrion via the carnitine shuttle. Thereafter, acyl-coA undergoes β-oxidation whereby a series of chain length specific enzymes progressively shorten it by two carbon atoms (= one acetyl-CoA). The resulting two-carbon acetyl-CoA either enters the Kreb’s (tricarboxylic acid) cycle or is converted to ketone bodies, 3-OHB and acetone (Figure 1).
Fatty acid oxidation disorders are caused either by mutations affecting the function of transport proteins involved in the carnitine shuttle, or the enzymes required for β-oxidation or ketogenesis. In these disorders a catabolic state precipitates hypoketotic hypoglycaemia as a consequence of impaired ketone body production coupled with impaired gluconeogenesis. The latter is a consequence of low acetyl-CoA.
The vast majority of cases present in late infancy as encephalopathy accompanied by signs of liver failure and hyperammonaemia. In long chain fatty acid oxidation disorders the presentation may also include acute cardiomyopathy. In attenuated forms presenting in adolescence or adulthood, hypoglycaemic encephalopathy may remain a feature of these conditions but catabolic stress is more commonly accompanied by recurrent rhabdomyolysis, chronic skeletal myopathy (pain, weakness) with or without cardiomyopathy.
In the acutely unwell individual, a fatty acid oxidation disorder can be readily identified by simultaneous determination of plasma concentrations of free fatty acids (FFA) and ketones (3-OHB) which reveals a high FFA to ketone ratio. There may be little to distinguish between the different fatty acid oxidation disorders on their clinical presentations, but the acylcarnitine profile is often diagnostic. Additional clues to the individual diagnoses are sought from the urine organic acid profile and plasma carnitine concentration. The inclusion of acylcarnitine analysis by tandem mass spectrometry into the newborn screening programme in the UK and elsewhere has led to an increased identification of presymptomatic cases many of which have a mild phenotype.
Acute management of fatty acid oxidation disorders requires administration of high dose glucose orally as a glucose polymer if tolerated, otherwise intravenously as a 10% glucose solution, aiming to maintain a blood glucose level greater than 5.5 mmol/L. Occasionally insulin needs to be co-administered to prevent severe hyperglycaemia and reverse the catabolic state. Long term management centres around avoidance of precipitants of catabolism, notably prolonged fasting such as may occur during times of intercurrent illness or unaccustomed intense or prolonged exercise. Anticipated events associated with catabolic stress such as elective surgery or childbirth should be managed prospectively with intravenous glucose. Some older anaesthetic agents administered as lipid suspensions (e.g., propofol) should be avoided in patients with these conditions.
Disorders of carnitine transport
These are disorders affecting transport of carnitine across the plasma membrane or acyl-coA esters across the mitochondrial membrane. The disorders comprise carnitine palmitoyltransferase 1 (CPT1) deficiency, CPT2 deficiency and carnitine transporter defect (CTD).
CPT1 can be distinguished biochemically from other fatty acid oxidation disorders by an elevated free and total plasma carnitine concentration coupled with universally low acylcarnitines.
CPT2 is one of the commonest IMDs to present in adulthood. There is a well-documented attenuated phenotype presenting as exercise intolerance and episodic rhabdomyolysis. Acylcarnitine profile shows an increased ratio of long chain 18- and 16-hydrocarbon fatty acids (C18 and C16) to short chain (C2) fatty acids. In addition, there is a low free carnitine concentration owing to increased renal excretion, with or without presence of non-specific dicarboxylic acids detectable on urine organic acid analysis. The diagnosis is confirmed by mutation analysis of the CPT2 gene - at least 60% of late-onset presentations are accounted for by the ‘common’ S113L mutation (3).
CTD is caused by a defect in the carnitine transporter responsible for carnitine uptake across the plasma membrane. A diagnosis of CTD is suspected if the free and total plasma carnitine concentration is profoundly low and all acylcarnitine compounds are also low. CTD is treatable with administration of high dose oral carnitine, and failure to take carnitine regularly can cause acute cardiomyopathy and sudden death.
Disorders of β-oxidation
These are named according to where in the β-oxidation chain the enzyme defect occurs. In very long-chain acyl-coA dehydrogenase (VLCAD) deficiency defective oxidation of 14-hydrocarbon chain (C14) to 12-hydrocarbon chain (C12) hydrocarbon fatty acids produces an acylcarnitine profile characterised by a high C14 proximal to the enzyme defect with low C12 distal to it and high ratio of C14 to C12. Urinary excretion of C6-C14 dicarboxylic acids is detectable on urine organic acid analysis.
After catalysis by VLCAD, long chain fatty acids undergo metabolism by the mitochondrial trifunctional protein (MTP) comprising four α-subunits mediating hydratase and dehydrogenase activity. Complete MTP deficiency is very rare and typically fatal in infancy. More commonly an isolated defect in the component mediating dehydrogenase activity, long-chain 3-hydroxyacy-CoA dehydrogenase (LCHAD), gives rise to a milder condition recognised by accumulation of long chain hydroxy acylcarnitine compounds and urinary excretion of C6 to C14 hydroxydicarboxylic acids. An adult presentation has been reported (4). Pregnant women who are heterozygous for LCHAD (or MTP) deficiency carrying an affected (homozygous) foetus are at high risk of HELLP syndrome (haemolysis, elevated liver enzymes, low platelets) and acute fatty liver of pregnancy (5).
After undergoing long chain β-oxidation, the resulting medium chain fatty acids are metabolised by medium chain acyl-coA dehydrogenase (MCAD). MCAD deficiency results in prominent accumulation of C8 and to a lesser extent C10 and C6 acylcarnitines with a high C8/C10 and C8/C2 ratio. There is a characteristic pattern of urinary excretion of C6 to C10 dicarboxylic acids identifiable on the urine organic acid profile. MCAD deficiency is by far the most common fatty acid oxidation disorder with a prevalence of 1:6,000 in the Northern European population, attributable to a prevalent mutation (c.985A>G) in the ACADM gene (6). Eighty percent of symptomatic cases in the Caucasian population carry at least one c.985A>G mutation, whilst in its homozygous state it accounts for 60% of asymptomatic patients detected by newborn screening (7,8).
A neuromyopathic phenotype dominates the clinical picture in attenuated forms of MCAD, VLCAD and LCHAD. The introduction of acylcarnitine analysis to the newborn screening programme in many countries has led to an increased incidence of milder forms of these conditions diagnosed in neonates who may have previously presented in adolescence or adulthood, or even not at all (6).
Short chain acyl-CoA dehydrogenase (SCAD) deficiency is recognisable biochemically as increased C4 acylcarnitines, usually detected incidentally and not associated with clinical symptoms. It may be a so-called ‘non-disease’. On the other hand short chain hydroxyacyl-CoA dehydrogenase (SCHAD) deficiency is a cause of congenital hyperinsulinaemic hypoglycaemia, the pathophysiology of which is independent of β-oxidation and responds to diazoxide. Adult onset forms have not been reported.
Multiple acyl-CoA dehydrogenase deficiency (MADD), also known as glutaric aciduria type 2, results from defects of electron transfer flavoprotein (ETF) or ETF-coenzyme Q-oxidoreductase (ETFQO). ETF and ETFQO transfer electrons from multiple flavin adenine dinucleotide (FAD)-dependent dehydrogenases to the respiratory chain. Defects affect the function not only of the acyl-coA dehydrogenases involved in fatty acid oxidation but also the dehydrogenases of amino acid metabolism. Biochemically it is identified by elevated levels of all acylcarnitine compounds from C4 to C18 and urinary excretion of lactic, glutaric, ethylmalonic and dicarboxylic acids. It has a wide range of clinical severity, in its most severe form it is fatal in infancy, but attenuated forms can present in at any age with hypoglycaemia, liver dysfunction and proximal myopathy. Riboflavin, a cofactor for ETF, is successfully used in the treatment of some patients with milder forms of the disorder (9).
Disorders of ketogenesis
The final two steps in the generation of ketones from fatty acids involve the enzymes 3-hydroxy-3-methylglutaryl (HMG)-CoA synthase and HMG CoA lyase. The clinical presentation of both conditions is similar to that seen in fatty acid oxidation disorders. However, the aetiology of the hypoglycaemia is of excessive consumption of glucose to compensate for profound hypoketosis caused by the enzyme defect rather than impaired gluconeogenesis. In HMG-CoA synthase deficiency profound hypoketosis is accompanied by a normal acylcarnitine profile. HMG-CoA lyase deficiency is recognisable by the presence of specific urinary metabolites (3-HMG, 3-methylglutaconic acid etc). An adult-onset presentation has only been reported for HMG-CoA lyase deficiency (10).
Glycogen storage disorders
Glycogen is a macromolecule comprised of up to 60,000 glucose residues joined in straight chains by α-1,4 linkages and at branches formed by α-1,6-linkages. It is predominantly found in liver where it serves as the main source of glucose for maintaining normoglycaemia between meals and is also abundant in muscle providing energy for muscle contraction. Numerous enzymes are involved in the synthesis and breakdown of glycogen and deficiencies of almost all have been identified as the causes of the glycogen storage disorders (GSD), denoted sequentially in order of their discovery as GSD I – XIII along with GSD 0 and Fanconi-Bickel syndrome. The clinical features of the GSDs depend on the somatic distribution of the glycogen affected by the enzyme defect. In ‘hepatic’ GSDs, impaired metabolised of liver glycogen results in hypoglycaemia with hepatomegaly. The GSDs exclusively affecting metabolism of muscle glycogen cause myopathy and/or cardiomyopathy.
Hepatic glycogen storage disorders causing hypoglycaemia are GSD I, III, VI, IX, GSD 0 (Figure 1) and Fanconi-Bickel syndrome. In GSD IIIa and IXb there are additional myopathic features. They usually present in infancy with hypoglycaemia, hepatomegaly caused by hepatic glycogen storage (except GSD 0) and growth retardation. Historically, clinical suspicion of a GSD diagnosis would be confirmed with a liver biopsy demonstrating glycogen-laden hepatocytes with abnormal activity of one of the GSD-causing enzymes. In the absence of a liver biopsy, skin fibroblasts could be used as an alternative tissue for enzyme analysis. Enzyme analysis did not allow for distinction between subtypes of a particular GSD and has largely now been superseded by mutation analysis.
The aim of treatment is to maintain normoglycaemia by avoidance of fasting. Children and some adults require overnight feeding with glucose polymer administered via a nasogastric or gastrostomy tube. Since the early 1980s uncooked cornstarch has been used to in addition to frequent meals and overnight feeds to bridge the gap between meals (11). Cornstarch provides a source of ‘slow release’ glucose through the action of amylase and therefore its metabolism doesn’t depend on the activity of any of the enzymes of glycogenolysis deficient in the hepatic GSDs. Its use has transformed the outlook for people with GSD, especially GSD I, by aiding growth and development in children, progression of puberty in adolescents and protecting against an acute metabolic crisis precipitated by fasting or illness at any age.
Glycogen Storage Disorder Type I
GSD I was the first GSD to be described. It is still occasionally referred to as von Gierke’s disease after the name of its discoverer. It comprises GSD Ia and GSD Ib. The incidence of GSD I is approximately 1 in 100,000 of whom about 1 in 8 have GSD Ib.
The metabolic pathways of both glycogenolysis and gluconeogenesis converge at the substrate, glucose-6-phosphate. Glucose-6-phosphate is dephosphorylated to glucose in the endoplasmic reticulum by a process involving the enzyme glucose-6-phosphatase and several transport systems. GSD Ia is caused by deficiency of glucose-6-phosphatase and GSD Ib, is caused by a defect in glucose-6-phosphate transporter on the endoplasmic reticulum. Because the final step in the generation of glucose from both glycogenolysis and gluconeogenesis is impaired, the associated hypoglycaemia in GSD I is profound. Individuals usually present in infancy with hepatomegaly and symptoms of fasting intolerance evident as soon as exogenous (dietary) sources of glucose are depleted. Brain development is normal if hypoglycaemic brain damage is averted. Poor fasting tolerance with an incumbent risk of hypoglycaemia persists throughout life though may be somewhat ameliorated with age by the appearance of an extra-hepatic glucose-6-phosphate hydrolase during adolescence (12). Many adults do not require continuous overnight feed but must still wake during the night to take cornstarch. Individuals of all ages remain at risk of metabolic decompensation during intercurrent illness if glucose intake is not maintained, the consequences of which include seizures, encephalopathy and life-threatening metabolic (lactic) acidosis.
Long-term, adults almost universally develop hepatic adenomas but these are particularly prevalent and develop at an earlier age if metabolic control has been poor. Their malignant transformation to hepatocellular carcinoma is a persistent serious complication that must be monitored for. Liver transplantation fully corrects the genetic defect and is potentially curative for both hypoglycaemia and long-term hepatic complications.
Additional features of GSD I arise from the shunting of unmetabolised substrates upstream of the enzyme defect through alternative pathways. Notably, lactate is produced from excess glucose-6-phosphate via the glycolytic pathway, a process under hormonal control which is enhanced when exogenous glucose is unavailable. It is also generated by continued ingestion of carbohydrates such as sucrose, fructose and lactose whose metabolism to glucose depends on glucose-6-phosphatase. In adults, hypoglycaemic awareness may be lost and lactate measurement, undertaken as part of the routine monitoring, provides a useful indicator of chronic hypoglycaemia and adequacy of dietary management.
Lactate competitively inhibits renal urate excretion resulting in hyperuricaemia and an increased risk of gout (13). It also provides an alternative source of energy for the brain protecting the individual to some extent from neuroglycopenic symptoms (14).
A mixed pattern hyperlipidaemia (high cholesterol and triglycerides) is commonplace in GSD I and is derived both from metabolism of excess acetyl-CoA via malonyl-CoA and reduced uptake and lipolysis of circulating lipoproteins (15). It is not known whether the hyperlipidaemia gives rise to an excess risk of cardiovascular disease, but in the UK, treatment with lipid lowering agents from a young age is part of standard of care. Excess malonyl-CoA also impairs mitochondrial β-oxidation and ketogenesis making it the only GSD causing hypoketotic hypoglycaemia (16). The potential additive effect of hypoketosis with hypoglycaemia on brain energy supply may in part be offset by high lactate as an alternative fuel for the brain.
GSD Ib is additionally complicated by neutropenia with variable neutrophil dysfunction, the latter predisposing to recurrent staphylococcal, streptococcal and E.coli bacterial infections and only partly ameliorated by administration of exogenous granulocyte colony-stimulating factor (GCSF). Neutropenia is also implicated in the pathogenesis of the ulceration of the mouth and small intestine resembling inflammatory bowel disease which develops during adolescence and is a major cause of morbidity. The aetiology of the neutropenia has recently been elucidated (17). Glucose-6-phosphate transporter protein is ubiquitously expressed, and in extra-hepatic tissues functions to eliminate 1,5 anhydroglucitol-6-phosphate (1,5AG6P), a close structural analogue of glucose-6-phosphate derived from polyol compounds present in food, via its precursor,1,5AG. In GSD Ib, defective glucose-6-phosphate transporter protein causes accumulation of 1,5AG6P which is a natural inhibitor of the first stage of glycolysis. This has particular consequences for neutrophil survival because they derive almost all of their energy from glycolysis and results in neutropenia.
A progressive proteinuric nephropathy develops in GSD Ib, the natural history of which is strikingly similar to diabetic nephropathy. The similarity may be accounted for by the presence of high levels of 1,5AG in both conditions. 1,5AG, the precursor to 1,5AG6P, competes for glucose in the tubules. Elimination of 1,5AG is enhanced by administration of sodium-glucose transporter inhibitors (SGLTi), otherwise known as the ‘gliflozins’ (18) and this class of drug is already proving to be effective in slowing the progression of diabetic nephropathy in diabetics independent of its glycaemic effect (19). By aiding the elimination of 1,5AG and thereby also preventing accumulation of 1,5AG6P, SGLTi drugs now offer an exciting prospect for treatment for both GSD Ib-associated neutropenia and kidney disease.
Glycogen Storage Disorder Type III
GSD III (Cori or Forbes disease) is caused by deficiency of glycogen debranching enzyme (amylo-1,6-glucosidase) required for the conversion of glycogen to glucose-1-phosphate in the first stage of glycogenolysis. Glucose-1-phosphate is in turn converted to glucose-6-phosphate and then to glucose. About 85% of individuals have a generalised enzyme defect affecting its activity in liver, skeletal muscle and heart, termed GSD IIIa. In GSD IIIb variant splicing of the enzyme results in a selective defect of the hepatic isoform. In both forms, ketotic hypoglycaemia accompanied by hepatomegaly is evident in the first year of life and may be clinically indistinguishable from GSD I, though as gluconeogenesis is unaffected, hypoglycaemia is typically milder and can be ameliorated by a high protein intake alongside cornstarch. Lactate and uric acid are usually normal. Hypoglycaemia improves with age and adults with GSD III rarely require overnight gastrostomy feeding. Conversely, in GSD IIIa, complications of myopathy dominate the clinical picture from the fourth decade. Muscle biopsy, if performed, demonstrates the presence of glycogen-laden vacuoles which accumulate over time to gradually replace muscle tissue causing a progressive skeletal myopathy and in many cases cardiomyopathy. A high protein diet is favoured by patients not only to ameliorate hypoglycaemia but also for its potential to build muscle and slow the progression of myopathy, though definitive evidence for this particular benefit has yet to be demonstrated. Hepatic fibrosis, cirrhosis and adenomata occur in a substantial minority of adults and should be screened for. Liver transplantation is not curative as it does not correct the muscle or cardiac complications.
Glycogen storage disorder type VI and IX
GSD VI (Hers disease) is caused by defective activity of liver glycogen phosphorylase. Liver phosphorylase works in conjunction with debranching enzyme (see section on GSD III) to metabolise the straight chains of glycogen to glucose-1-phosphate in the first step of glycogenolysis.
GSD IX is caused by phosphorylase kinase deficiency (PHK). PHK is expressed in liver and muscle, the four hepatic forms are GSD IX a-d. GSD IXb has additional myopathic features. Overall, GSD IX is the commonest GSD and GSD IXa is the commonest subtype. GSD IXa and GSD IXd are distinguished from all other disorders discussed in this article by their X-linked inheritance pattern.
PHK deficiency in GSD IX results in a failure to transform liver (or muscle) glycogen phosphorylase into its active form (phosphorylase a) for degradation of glycogen. The enzyme defect affects the last step in the cascade of metabolic reactions initiated by glucagon in liver (and adrenaline in muscle) in response to hypoglycaemia or an energy demand.
Like other hepatic GSDs, both conditions present in childhood with ketotic hypoglycaemia and growth retardation. Hepatomegaly is accompanied by raised liver transaminases and hyperlipidaemia. The clinical course of both conditions is similar. Hypoglycaemia is typically milder than in GSD I and III but suppression of ketosis with frequent meals supplemented with cornstarch between meals nevertheless has benefits to growth and development. The dietary regimen can usually be relaxed in adulthood. Hepatomegaly resolves in adolescence and hepatic adenomas are rare.
Glycogen storage disorder type 0
GSD 0 is caused by deficiency of the hepatic glycogen synthase resulting in impaired hepatic glycogen synthesis. Because the hepatic storage of glycogen is affected, there is no hepatomegaly. However, it is classified as a GSD because hypoglycaemia is caused by a lack of glycogen as a source of glucose during fasting. Paradoxically there is also post-prandial hyperglycaemia because excess dietary glucose cannot be assimilated into glycogen and individuals are sometimes misclassified as having diabetes (20). Like the other GSDs, treatment is aimed at maintaining normoglycaemia. Its relatively benign course and the absence of hepatic glycogen storage may mean that it is underdiagnosed (21).
Fanconi-Bickel syndrome
Fanconi-Bickel syndrome (classified as GSD XI) is caused by a defect in the glucose transporter, GLUT2. In hepatocytes GLUT2 acts as a glucose sensor to stimulate glucose release from glycogen in response to a low extracellular glucose level. This process fails in Fanconi-Bickel syndrome leading to inappropriate hepatic glycogen synthesis and storage coupled with inhibition of glycogenolysis and gluconeogenesis. At the renal tubules GLUT2 transports glucose out of cells and defective GLUT2 activity leads to renal tubular accumulation of glycogen. This causes a Fanconi-type tubulopathy characterised by severe glycosuria (which may contribute to the hypoglycaemia), renal tubular acidosis, phosphaturia (causing hypophosphataemic rickets) and amino aciduria.
As with other GSDs, treatment is aimed at maintenance of normoglycaemia. Additionally, hypophosphataemic rickets is treated with phosphate and vitamin D supplementation. It may be necessary to correct acidosis by administering bicarbonate. In adulthood, the clinical picture is dominated by complications of the tubulopathy rather than hypoglycaemia. Hepatic adenomas have not been observed.
Disorders of gluconeogenesis
Recurrent clinically significant hypoglycaemia is a feature of disorders of gluconeogenesis caused by enzyme defects immediately upstream of glucose in the gluconeogenesis pathway. These are GSD I (discussed above) and fructose-1,6-bisphosphatase deficiency (Figure 1). Defect of an enzyme closer to the Krebs cycle, notably pyruvate carboxylase deficiency which converts pyruvate to oxaloacetate, typically causes neurodegenerative disease and lactic acidosis more akin to the mitochondrial disorders.
Fructose-1,6-bisphosphatase deficiency
Fructose-1,6-bisphosphatase (FBPase) is responsible for the conversion of fructose-1,6-bisphosphate to fructose-6-phosphate which is the immediate precursor to glucose-6-phosphate. In FBPase deficiency, glycogenolysis is intact hence individuals are most at risk of hypoglycaemia only when hepatic glycogen stores are depleted during fasting (or when they’re limited such as in the neonatal period). A large fructose intake can also precipitate hypoglycaemia because of its rapid conversion to fructose-1-phosphate which is a natural inhibitor of glycogenolysis. The diagnosis is suspected by an elevated lactate and ketones with 2-oxoglutaric acid detected on urine organic acid analysis. Confirmation of the diagnosis is by mutation analysis. Treatment is aimed at maintaining normoglycaemia by avoidance of fasting and limiting fructose intake. Hypoglycaemia is readily corrected by oral or intravenous glucose, and the clinical course is benign provided severe hypoglycaemia is averted.
IMDs causing post-prandial hypoglycaemia in adulthood
The causes and investigation of postprandial (reactive) hypoglycaemia in adulthood is discussed in detail elsewhere in this issue. Those classed as IMDs include HFI and three of the CDG.
Hereditary fructose intolerance
HFI is an inherited defect of aldolase B activity with an incidence of approximately 1:20,000 in the European population. Aldolase B catalyses cleavage of fructose-1-phosphate into phosphorylated C3-metabolites that enter glycogenolysis or gluconeogenesis (Figure 1). In patients with HFI ingestion of fructose causes accumulation of fructose-1-phosphate which inhibits hepatic glycogenolysis and gluconeogenesis and depletes adenosine triphosphate (ATP) causing post-prandial hypoglycaemia along with vomiting and abdominal pain. Continued ingestion of fructose over a long period results in progressive liver dysfunction, hepatomegaly, and renal tubular dysfunction. Milder forms of the condition may go unrecognised until adulthood. A cardinal feature of the condition in adults is excellent dentition as a consequence of lifelong aversion to sweet foods. Associated biochemical features arise from renal tubular damage causing glycosuria, albuminuria, aminoaciduria and presence of urinary reducing substances. The most comprehensive review of the condition is still that dating from 1998 by Ali et al. (22).
The key to the diagnosis is recognition of the association of symptoms with ingestion of fructose-containing foods and a positive effect from withdrawal of these foods from the diet. Confirmation of the diagnosis may be sought from mutation analysis of the ALDOB gene though occasionally a liver biopsy for enzyme activity is necessary. Fructose challenge is no longer recommended even under medical supervision.
The hepatic and renal tubular complications of the condition are reversible by complete withdrawal of fructose from the diet. Fructose is present in large amounts not only in fruits and some vegetables, but also as a component of table sugar as the glucose/fructose disaccharide, sucrose, and in food additives as high fructose corn syrup (known in the EU as glucose-fructose corn syrup) and sorbitol which is metabolised via fructose. Specialist dietetic support is advisable to help patients identify the common, and less obvious, sources of dietary fructose and to achieve a nutritionally replete diet despite exclusion of fruit and many vegetables. Prognosis is excellent with adherence to dietary advice.
Congenital disorders of glycosylation
Many enzymes, transport and membrane proteins and hormones require glycosylation to render them fully functional as glycoproteins. CDGs are characterised by disturbance of various steps in the biosynthesis of glycoprotein carbohydrate side chains (glycans) and the resulting glycoprotein dysfunction produces a broad spectrum of clinical symptoms. Traditionally CDG were classified as either CDG Type I caused by defects in assembly of the glycan or its transfer to the associated protein in the endoplasmic reticulum or CDG Type II caused by defects in the processing of the protein-bound glycans in the Golgi body. Latterly though, as the genetic mutations causing the CDG subtypes have been elucidated, they have been reclassified by their gene name.
Three subtypes, phosphomannomutase 2 deficiency, termed PMM2-CDG (formerly CDG 1a), phosphomannose isomerase deficiency, PM1-CDG (formerly CDG Ib) and α1,3-mannosyl transferase deficiency (ALG3-CDG, formerly CDG Id) are associated with post-prandial hyperinsulinaemic hypoglycaemia. Hypoglycaemia never occurs in isolation in these conditions, but rather as part of a clinical syndrome and the presence of associated features should prompt further investigation. The conditions are screened for by isoelectric focussing (IEF) of transferrin. In all subtypes formerly categorised as Type I, which includes the three causing post-prandial hypoglycaemia, the IEF picture is of elevated disialotransferrin and asialotransferrin bands with a decrease in tetrasialotransferrin. The precise subtype can be inferred from the associated clinical features and confirmed with mutation or enzymatic analysis.
Of the three that cause hypoglycaemia, PMM2-CDG is by far the most common and indeed accounts for 80% of all known cases of CDG. The clinical syndrome invariably includes dysmorphism and variable intellectual disability (23). A milder adult phenotype is recognised (24) in which neurological symptoms, hypogonadism and complications of coagulopathy predominate. The author is aware of three adults diagnosed in the last two years in the UK via the 100,000 genomes project. The hypoglycaemia in PMM2-CDG may be relatively mild, especially in attenuated forms of the disorder, and thus escape further investigation in childhood. Hypoglycaemia responds well to diazoxide.
PM1-CDG is characterised by protein losing enteropathy, hepatic fibrosis and hypoglycaemic episodes which range from mild to severe, or even lethal. There is no associated dysmorphism or intellectual disability (25). The wide spectrum of severity and absence of intellectual disability makes it conceivable that milder cases go undetected until later in life when complications of hepatic fibrosis may predominate. Hypoglycaemia and protein-losing enteropathy can be ameliorated with administration of oral mannose making it the only potentially treatable CDG (26,27).
ALG3-CDG is an exceptionally rare cause of severe hyperinsulinaemic hypoglycaemia presenting in infancy and associated with β-cell hyperplasia (28). Adult presentations have not been reported.
Summary
Hypoglycaemia is a common presenting problem at all ages. However, in adults, it is significantly more likely to be of an endocrine rather than genetic aetiology. Once non-IMD causes have been excluded, an appreciation of whether clinical symptoms occur in the fasting or post-prandial state aids diagnosis. Thereafter the presence of additional clinical features and identification of biochemical abnormalities with a combination of routine and IMD-oriented screening investigations will facilitate a diagnosis. The increased availability of confirmatory genetic testing in recent years has enhanced our understanding of the relative pathogenicity of specific mutations, a consequence of which is the recognition of attenuated phenotypes of disorders historically considered to be paediatric conditions. In these attenuated forms hypoglycaemia may persist into adulthood but is generally milder and other clinical and biochemical features predominate.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Rousseau Gama) for the series “Adult Spontaneous Hypoglycaemia” published in Journal of Laboratory and Precision Medicine. The article has undergone external peer review.
Reporting Checklist: The author has completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/jlpm-20-100
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm-20-100). The series “Adult Spontaneous Hypoglycaemia” was commissioned by the editorial office without any funding or sponsorship. The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2009;94:709-28. [Crossref] [PubMed]
- Saudubray JM, van den Berghe G, Walter J. Inborn Metabolic Diseases: Diagnosis and Treatment. Fifth edition. Berlin: Springer Verlag, 2012.
- Taroni F, Verderio E, Dworzak F, et al. Identification of a common mutation in the carnitine palmitoyltransferase II gene in familial recurrent myoglobinuria patients. Nat Genet 1993;4:314-20. [Crossref] [PubMed]
- Schaefer J, Jackson S, Dick D, et al. Trifunctional enzyme deficiency: adult presentation of a usually fatal beta-oxidation defect. Ann Neurol 1996;40:597-602. [Crossref] [PubMed]
- Wilcken B, Leung K, Hammond J, et al. Pregnancy and fetal long chain 3-hydroxyacyl coenzyme A dehydrogenase deficiency. Lancet 1993;341:407-8. [Crossref] [PubMed]
- Andresen B, Dobrowolski S, O’Reilly L, et al. Medium chain acyl-CoA dehydrogenase (MCAD) mutations identified by MS/MS-based prospective screening of newborns differ from those observed in patients with clinical symptoms: identification and characterisation of a new prevalent mutation that results in mild MCAD deficiency. Am J Hum Genet 2001;68:1408-18. [Crossref] [PubMed]
- Wilcken B, Haas M, Joy P, et al. Outcome of neonatal screening for medium-chain acyl-CoA dehydrogenase deficiency in Australia: a cohort study. Lancet 2007;369:37-42. [Crossref] [PubMed]
- Waddell L, Wiley V, Carpenter K, et al. Medium-chain acyl-CoA dehydrogenase deficiency: Genotype–biochemical phenotype correlations. Mol Genet Metab 2006;87:32-9. [Crossref] [PubMed]
- Olsen RK, Olpin SE, Andresen BS, et al. ETFDH mutation as a major cause of riboflavin-responsive multiple acyl-CoA dehydrogenation deficiency (MADD). Brain 2007;130:2045-54. [Crossref] [PubMed]
- Reimao S, Morgado C, Almeida IT, et al. 3-Hydroxy-3-methylglutaryl-coenzyme A lyase deficiency: initial presentation in a young adult. J Inherit Metab Dis 2009;32:S49-52. [Crossref] [PubMed]
- Chen Y, Cornblath M, Sidbury J. Cornstarch therapy in type I glycogen-storage disease. N Engl J Med 1984;310:171-5. [Crossref] [PubMed]
- Shieh J, Pan C, Mansfield B, et al. A glucose-6-phosphate hydrolase, widely expressed outside the liver, can explain age-dependent resolution of hypoglycaemia in glycogen storage disease type Ia. J Biol Chem 2003;278:47098-103. [Crossref] [PubMed]
- Cohen J, Vinik A, Faller J, et al. Hyperuricemia in glycogen storage disease type I. Contributions by hypoglycaemia and hyperglucagonemia to increased urate production. J Clin Invest 1985;75:251-7. [Crossref] [PubMed]
- Boumezbeur F, Petersen K, Cline G, et al. The contribution of blood lactate to brain energy metabolism in humans measured by dynamic 13C nuclear magnetic resonance spectroscopy. Neuroscience 2010;30:13983-91. [Crossref] [PubMed]
- Bandsma RH, Prinsen BH, van Der Velden Mde S, et al. Increased de novo lipogenesis and delayed conversion of large VLDL into intermediate density lipoprotein particles contribute to hyperlipidemia in glycogen storage disease type 1a. Pediatr Res 2008;63:702-7. [Crossref] [PubMed]
- Binkiewicz A, Senior B. Decreased ketogenesis in von Gierke’s disease (type 1 glycogenosis). J Pediatr 1973;83:973-8. [Crossref] [PubMed]
- Veigha-da-Cunha M, Chevalier N, Stephenne X, et al. Failure to eliminate a phosphorylated glucose analog leads to neutropenia in patients with G6PT and G6PC3 deficiency. PNAS 2019;116:1241-50. [Crossref] [PubMed]
- Balis DA, Tong C, Meininger G. Effect of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, on measurement of serum 1,5-anhydroglucitol. J Diabetes 2014;6:378-80. [Crossref] [PubMed]
- Heerspink H, Desai M, Jardine M, et al. Canagliflozin slows progression of renal function decline independently of glycemic effects. J Am Soc Nephrol 2017;28:368-75. [Crossref] [PubMed]
- Weinstein DA, Correia CE, Saunders AC, et al. Hepatic glycogen synthase deficiency: an infrequently recognised cause of ketotic hypoglycaemia. Mol Genet Metab 2006;87:284-8. [Crossref] [PubMed]
- Bachrach B, Weinstein D, Orho-Melander M, et al. Glycogen synthase deficiency (glycogen storage disease type 0) presenting with hyperglycaemia and glucosuria: report of three new mutations. J Pediatr 2002;140:781-3. [Crossref] [PubMed]
- Ali M, Rellos P, Cox TM. Hereditary fructose intolerance. J Med Genet 1998;35:353-65. [Crossref] [PubMed]
- Grünewald S. The clinical spectrum of phosphomannomutase 2 deficiency (CDG-Ia). Biochimica et Biophysica Acta 2009;1792:827-34. [Crossref] [PubMed]
- Marie-Lorraine M, Mignot C, De Lonlay P, et al. 29 French adult patients with PMM2-congenital disorder of glycosylation: outcome of the classical pediatric phenotype and depiction of a late-onset phenotype. Orphanet J Rare Dis 2014;9:207-15. [Crossref] [PubMed]
- de Lonlay P, Seta N. The clinical spectrum of phosphomannose isomerase deficiency, with an evaluation of mannose treatment for CDG-Ib. Biochim Biophys Acta 2009;1792:841-3. [Crossref] [PubMed]
- Niehues R, Hasilik M, Alton G, et al. Carbohydrate-deficient glycoprotein syndrome type Ib. Phosphomannose isomerase deficiency and mannose therapy. J Clin Invest 1998;101:1414-20. [Crossref] [PubMed]
- Harms H, Zimmer K, Kurnik K, et al. Oral mannose therapy persistently corrects the severe clinical symptoms and biochemical abnormalities of phosphomannose isomerase deficiency. Acta Paediatr 2002;91:1065-72. [Crossref] [PubMed]
- Sun L, Eklund EA, Chung WK, et al. Congenital disorder of glycosylation id presenting with hyperinsulinemic hypoglycemia and islet cell hyperplasia. J Clin Endocrinol Metab 2005;90:4371-5. [Crossref] [PubMed]
Cite this article as: Dawson C. Inherited metabolic disorders associated with hypoglycaemia in adulthood: a narrative review. J Lab Precis Med 2021;6:19.


