
Autoimmune hypoglycaemia: a narrative review from the laboratory perspective
Introduction
Immunity is crucial in maintaining health. Immunity can, however, go wrong; a key example is autoimmunity, in which the immune system reacts to self-antigens. The diseases and presentations that can occur as a result of autoimmunity are wide ranging and, although rare, include autoimmune hypoglycaemia.
Hypoglycaemia is a relatively common occurrence in diabetic patients once they commence treatment with insulin or insulinotropic drugs. Other broad causes of hypoglycaemia in adults include drugs, critical illness, hormone deficiencies and endogenous hyperinsulinism (1). Two autoimmune conditions, insulin autoimmune syndrome (IAS) and type B insulin resistance (TBIR), are rare causes of spontaneous hypoglycaemia. IAS is the result of insulin autoantibodies (IAA), and TBIR the result of insulin receptor antibodies; both may result in spontaneous hypoglycaemia due to inappropriate endogenous hyperinsulinaemia. Whilst the literature yields case reports and reviews on autoimmune hypoglycaemia, these tend not to have a strong focus on the clinical laboratory and methodology used in diagnosis. As such, this short review will describe the pathophysiology of both conditions, and consider how the laboratory contributes to diagnosis and management of these patients. We present the following article in accordance with the narrative review reporting checklist (available at https://dx.doi.org/10.21037/jlpm-20-111).
Methodology
Papers included in this literature review were identified using a search of PubMed. Literature was included from the first published case of IAS in 1972 until 2020. All published case reports, studies and literature reviews were considered in producing this article. There are limited large-scale studies due to the rare nature of the conditions discussed.
Insulin and insulin receptor antibodies
Autoantibodies to endogenous insulin or the insulin receptor are considered sine qua non for the diagnosis of autoimmune hypoglycaemia (2).
Insulin antibodies (IA) developing as a result of exogenous insulin administration are common, polyclonal in origin, and development depends on age, insulin type, and route of insulin delivery. IA also demonstrate association with histocompatibility leucocyte antigen (HLA) types -B15, -DR4 and -DR7 (3). They are usually not clinically significant and are of low affinity (4). High titres of IgE IA are, however, often present in patients with type 1 insulin allergy (5).
IAA, which occur in people not previously exposed to exogenous insulin, are considered less common. The prevalence of IAA in the adult general population has not been systematically studied. They may be present in high titres and be of high affinity, and thus have the potential to be clinically significant (4). Whilst IAA are mostly clinically insignificant, they are considered a risk factor for developing type 1 diabetes (6-8), and rarely may be a cause of post-prandial hypoglycaemia (IAS) (9).
Autoantibodies against the insulin receptor (IRA) are predominantly polyclonal IgG (10), and appear to cause disease mainly in black middle-aged women with pre-existing autoimmune disease, but may also be associated with infectious and lymphoproliferative disease (11,12). IRA may cause TBIR syndrome, which typically presents with hyperglycaemia due to insulin resistance, but may also present with hypoglycaemia or hyperglycaemia-hypoglycaemia depending on insulin receptor inhibition or activation (11,12).
Although IA and IAA may not have pathological significance, they have the potential to cause variable interference in not only insulin immunoassays but also proinsulin and C-peptide immunoassays (13). The possibility of immunoassay interference should, therefore, always be considered in the laboratory investigation of hypoglycaemia.
In summary, antibodies to insulin and its receptor may be of no pathological, clinical or immunoassay significance, or be of no pathological significance but be a source of erroneous laboratory results giving rise to clinical confusion, or may be of pathological significance by causing autoimmune hypoglycaemia. Identification of an autoimmune cause for hypoglycaemia is important to ensure the patient receives the correct diagnosis and appropriate treatment. It is, therefore, vital that clinicians are aware of both the laboratory tests available, and also the potential for laboratory interference, when investigating hypoglycaemia.
IAS: an introduction
The cardinal features of IAS are non-ketotic endogenous hyperinsulinaemic hypoglycaemia, molar ratios of C-peptide:insulin of <1 (in the absence of severe renal disease), high IAA titres (without prior exposure to exogenous insulin) and identification of macroinsulin (2). Typical presentation is that of post-prandial hypoglycaemia, with onset in adulthood (9).
IAS: predisposing and trigger factors
IAS was first described in 1972 in a 47-year-old Japanese man with severe, spontaneous, hypoglycaemia (14). IAS is now reported to be the third most common cause of hypoglycaemia in Japan, after insulinoma and non-islet cell tumours (15). In the same year, IAS was also reported in a 40-year-old Norwegian man with reactive hypoglycaemia (16). IAS, however, remains a rare cause of hypoglycaemia in Caucasian populations. Frequently, IAS presents in patients with other autoimmune disorders, such as Graves’ disease (17) with a range in the severity of hypoglycaemia and its symptoms.
The geographical variability in prevalence is attributable to the fact that IAS is strongly associated with certain HLA-DR4 alleles, in particular, DRB1*04:06 (18). An additional trigger is usually present that precipitates development of IAS; these may broadly be split into infections or drug related triggers. Infection related triggers include mumps, Coxsackie B influenza, measles and hepatitis C, whilst drug related triggers include methimazole, carbimazole, propylthiouracil and alpha-lipoic acid, amongst others (2). Usually, drug-triggered IAS involves sulfhydryl containing drugs. Rajpal et al. reported a case of IAS in a 79-year-old white male following the commencement of clopidogrel which does not contain a sulfhydryl group, but its active metabolite does (19). The patient presented with recurrent episodes of hypoglycaemia, with inappropriately elevated total insulin and C-peptide levels, no evidence of insulinoma on imaging, and high anti-IAA titre. Post-polyethylene glycol (PEG) precipitation (free) insulin levels demonstrated appropriate insulin levels for the level of glycaemia, and discontinuation of clopidogrel and glucocorticoid therapy resulted in resolution of the hypoglycaemic episodes with dramatic reduction in total insulin and IAA levels (19).
Bresciani et al. reported a 70-year-old Italian woman with sudden onset of fasting and postprandial hypoglycaemic episodes following commencement of a multivitamin containing alpha-lipoic acid. Insulin and C-peptide were inappropriately elevated and extremely high titres of IA were detected. HLA typing demonstrated the presence of DRB1*04:06. Prednisone and diazoxide helped normalization of blood glucose levels and reduction in IAA (20). This may be of importance as alpha-lipoic acid is a widely available over-the-counter health supplement.
IAS has also been observed in patients with other autoimmune diseases including rheumatoid arthritis and systemic lupus erythematosus (SLE), and haematological diseases such as multiple myeloma (18).
The triggers of IAS are, therefore, varied and include genetic predisposition, infection, and drugs. These should all be considered when a diagnosis of IAS is made, as removal of the trigger, where possible, may be beneficial.
IAS: pathophysiology
The hypoglycaemia commonly occurs three to four hours post-prandially, following preceding post-prandial hyperglycaemia (18). It is generally accepted that the mechanism of hypoglycaemia is that following a meal, large amounts of insulin are bound by IAA, reducing insulin bioavailability. This causes hyperglycaemia, which further stimulates insulin release and binding. The insulin-antibody complexes are unstable and dissociate a few hours after eating, resulting in a large amount of free, active insulin, resulting in hypoglycaemia (19). Most hypoglycaemic episodes resolve within three to 6 months of diagnosis, particularly with discontinuation of the drug that triggered the IAS (18).
IAS has the potential to masquerade as insulinoma. Lohmann et al. reported a 69-year-old woman initially misdiagnosed with insulinoma as the cause of severe spontaneous hypoglycaemia. Investigations showed hypoglycaemia with elevated insulin, C-peptide and proinsulin; however, no tumour was identified during surgery. After surgery, further laboratory testing demonstrated discrepancy between insulin and C-peptide levels, leading to measurement of IAA, which were found to be elevated (21). This demonstrates the value of the C-peptide:insulin ratio in identifying IAS as discussed below.
IAS: laboratory features
IAS is identified by inappropriately high plasma insulin, proinsulin and C-peptide concentrations during hypoglycaemia, typically with a plasma C-peptide:insulin molar ratio of <1 and absence of ketosis. The presence of IAA is diagnostic of IAS and can be confirmed by the detection of macroinsulin using PEG precipitation and/or gel filtration chromatography (GFC) (22).
A high titre of IAA is essential in the diagnosis of IAS. Several immunoassay kits are available to detect insulin specific antibodies. IgG is the most common antibody type found in IAS, and often commercial kits only measure IgG. However, a negative test result does not rule out IAS, as rarely patients may present with different insulin specific Ig types, such as IgM or IgA (23). Insulin specific antibody immunoassays are not well standardised, and often require relatively large volumes of serum (24). Commercial assays described for measurement of insulin specific autoantibodies include a human insulin specific ImmunoCAP method to measure anti-insulin IgG or IgE and the Biomerica Isletest ELISA for serum anti-insulin IgG. Measurement of IAA testing is a first line investigation recommended by The American Endocrine Society in investigation of endogenous hyperinsulinaemic hypoglycaemia to identify IAS and prevent misdiagnosis (1).
Detection of IAA, however, does not prove the presence of the circulating IA complexes that underlie the pathophysiology of IAS (22). Several adjunctive approaches have been used to measure insulin-autoantibody complexes. The most common is to measure insulin before and after immunoprecipitation of Ig with PEG or ammonium sulphate (25). PEG precipitates all Ig including any insulin Ig complexes (IgG, IgM and IgA) present and is therefore non-specific and used as a preliminary test (26). This removes the insulin-autoantibody complexes and then only free available insulin is measured. A study also followed up PEG precipitation with GFC to identify the specific Ig type (22). The method sensitivity was enhanced by ex vivo addition of insulin (22). A problem with PEG precipitation is that it is not specific to all Ig and Ig complexes, and a proportion of the free analyte will be precipitated (26). GFC is the gold standard method for detecting macro-analyte complexes, e.g., antibody complexes. GFC, however is limited by dilution of the sample as it passes through the column therefore the complex must be present at high levels initially and also the dilution of samples passing through the column may disrupt the complex equilibrium. Measurement of insulin pre- and post-PEG precipitation and detection of macroinsulin complexes, are important in the confirmation of IAS.
The pancreatic secretion of insulin and C-peptide is equimolar but the half-life of insulin is much less than that of C-peptide. Circulating C-peptide concentrations are, therefore, greater than those of insulin, giving a C-peptide:insulin molar ratio of >1 in the absence of renal failure. In IAS, the binding of insulin to IAA prolongs the half-life of insulin such that circulating insulin concentrations exceed those of C-peptide, resulting in a C-peptide:insulin molar ratio of <1 (2). In other causes of endogenous hyperinsulinaemia the C-peptide:insulin ratio is >1. However, a C-peptide:insulin molar ratio of >1 does not exclude IAS, and its use in diagnosis of IAS has been criticised, due to wide variations in proinsulin and C-peptide concentrations in these patients, and potential immunoassay interference (27).
Specificities of immunoassays vary according to reagent antibody. The aim is to directly test all forms of endogenous insulin/proinsulin autoantibodies to exclude IAS, particularly in non-diabetic hypoglycaemic patients (23,28). Often, as a result of the IAA, insulin concentrations are excessively raised in IAS, beyond what may be expected in insulinoma, due to antibody cross-reactivity (9). The laboratory features of IAS are summarized in Table 1, and compared to the features of insulinoma, exogenous insulin use, and insulin secretagogue use, highlighting the key differences to be aware of when interpreting laboratory tests.
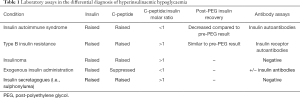
Full table
Autoimmune hypoglycaemia: due to IA and paraproteins
Autoimmune hypoglycaemia may rarely be due to IA and paraproteins with similar pathophysiological mechanisms as in IAS. As mentioned, IA often occur in patients treated with exogenous insulin, however these are usually present at low titres, and are of low affinity (4), and subsequently rarely cause hypoglycaemia.
Waldron-Lynch et al. reported a 63-year-old Caucasian man with spontaneous hypoglycaemia, with inappropriately high insulin, C-peptide and proinsulin levels and elevated aminotransferases. Urine sulfonylurea screen and imaging were both normal, and IAS was diagnosed. Serology diagnosed hepatitis C, and protein electrophoresis revealed a monoclonal IgGκ band with an additional IgGλ light chain band found to be capable of binding insulin. Treatment of the multiple myeloma resulted in remission of the IAS (4).
TBIR: an introduction
Insulin resistance is a common phenomenon with a range of causes, most frequently resulting in hyperinsulinaemia with or without hyperglycaemia. TBIR may be defined as altered insulin signaling as a result of insulin receptor autoantibodies (IRA). Its exact prevalence is not known, but TBIR is considered to be extremely rare, with black middle-aged women most commonly affected (11). TBIR was first described in 1975 in a small group of women (29), and named the following year (30). The most common presentation is periods of severe hyperglycaemia unresponsive to exogenous high-dose insulin treatment; however, TBIR may also result in hypoglycaemia (11). The hypoglycaemia may be post-prandial or fasting (31). Occasionally, hypoglycaemia may be the only presentation (32), but this is uncommon. Patients may switch between hyperglycaemia and hypoglycaemia (10). TBIR is reported to present with a range of other signs and symptoms, including weight loss, hyperandrogenism and acanthosis nigricans, and often presents in conjunction with paraneoplastic disease, rheumatological conditions, SLE and a range of other autoimmune dermatological and hepatic disorders (11,31). The prognosis of TBIR is variable; Arioglu et al. reported hypoglycaemic complications as a cause of death in several patients, but several patients also demonstrated spontaneous remission of TBIR (10).
TBIR: predisposing factors
Middle-aged, African-American women are predominantly affected (33). TBIR, however, has also been reported in children, the elderly, men and other ethnicities (10).
Arioglu et al. followed up 24 patients with TBIR over a period of 28 years, and reported that SLE was the most common autoimmune disease associated with IRA, with 46–50% of the patients having underlying SLE (10). TBIR titres correlate with SLE disease activity, with high titre IRA being identified in a 59-year-old patient with hyperglycaemia during an episode of active SLE (34). Insulin and plasma exchange were not effective in this patient, but induction of SLE remission through steroid and immunosuppressive therapy resulted in disappearance of IRA and improvement in glycaemic control.
Lebkowska et al. reported a 27-year-old man presenting with weight loss of 20 kg over a year and a past medical history of psoriasis and new onset of Raynaud’s phenomenon. He was anaemic and thrombocytopenic, with elevated glucose and HbA1c concentrations. He, however, developed asymptomatic fasting hypoglycaemia during the course of his admission. Subsequent investigations demonstrated IRA, and immunology showed high titres of anti-nuclear antibodies and anti-dsDNA antibodies, resulting in diagnosis of connective tissue disease (35). Sjoholm et al. reported TBIR in a 25-year-old Caucasian woman developing a few months after new-onset type 1 diabetes, with sudden deterioration in glycemic control despite high insulin doses (36). Rapid improvement was seen following treatment with prednisolone, and radio-labelled insulin binding to donor adipocytes incubated with patient serum demonstrated the presence of IRA.
Thus, whilst middle aged black females with autoimmune disease are most affected, it is important to consider the diagnosis in others regardless of age, gender or ethnicity.
TBIR: pathophysiology
The pathogenesis of TBIR is ill-understood. Limited patient studies have reported IRA to be polyclonal in nature and primarily IgG (10). The autoantibodies have demonstrated three different biological effects in vitro: mimic insulin activity, inhibit insulin binding to receptor, and desensitise tissues to insulin (32). The predominating effect determines whether the patient demonstrates hyperglycaemia or hypoglycaemia. It is generally accepted that high titres of IRA result in hyperglycaemia due to desensitisation of tissues, whilst low titres act as a receptor agonist resulting in hypoglycaemia (11). There is no set definition of TBIR, however, Willard et al. propose that a biochemical triad of elevated fasting insulin, hyperadiponectinaemia and low/normal fasting triglycerides, in a patient demonstrating both acanthosis nigricans and an autoimmune disease, may be considered a “working” definition (11). Patients generally require very high levels of exogenous insulin to control their hyperglycaemia. Measurement of IRA help confirm a diagnosis, with a systematic review reporting 83.2% of patients being IRA positive (12).
TBIR: laboratory features
Hypoglycaemia due to TBIR is identified by inappropriately high plasma insulin, proinsulin and C-peptide concentrations during hypoglycaemia, with plasma C-peptide:insulin molar ratios of >1, supported by hypotriglyceridaemia (37). Unlike IAS, measurement of insulin post-PEG precipitation does not usually change significantly from the pre-PEG result (see Table 1).
Measurement of IRA is beneficial; however, this assay is not routinely available. It may be measured at specialist research laboratories. In the UK, the assay is currently offered by the Supra-regional Assay Service at Addenbrookes Hospital Endocrinology Laboratory, in support of the NHS Severe Insulin Resistance service (38).
Conclusions
IAS and TBIR are rare, but important, causes of hypoglycaemia. Whilst both have an autoimmune basis, and both can result in hyperinsulinaemic hypoglycaemia, their pathophysiology, treatment and prognosis are different.
The classic presentation of IAS is endogenous hyperinsulinaemic hypoglycaemia, with high titre IAA, frequently following a trigger such as drugs or infection. IA complexes form, initially causing hyperglycaemia, followed by complex dissociation and subsequent hypoglycaemia. Patients often have a history of other autoimmune conditions, and HLA DRB1*04:06 is a risk factor for IAS. Detection of IAA, and a decreased post-PEG insulin measurement compared to pre-PEG measurement, supports the diagnosis. Rarely, hypoglycaemia may result due to presence of IA or paraproteins, through a similar mechanism.
TBIR is associated with hyperinsulinaemic hyperglycaemia resistant to insulin treatment, but patients may also develop fasting or post-prandial hypoglycaemia depending on the inhibiting or activating action of the IRA. TBIR more commonly affects women and is associated with other autoimmune conditions. Unlike IAS, post-PEG insulin measurement is not significantly different to pre-PEG measurement, and IRA are commonly detected in these patients.
IAS generally has a good prognosis once the precipitating factor is removed. TBIR, however, has a variable prognosis with significant mortality, and the development of hypoglycaemic episodes is an indicator of poor prognosis.
This review summarises the pathophysiology and laboratory features of autoimmune causes of hypoglycaemia, with an emphasis on laboratory testing. It highlights the importance of further laboratory investigations into hyperinsulinaemic hypoglycaemia, before subjecting the patient to potentially unnecessary pancreatic surgery, and also demonstrates the potential interferences in laboratory testing that clinicians should be aware of, in order to correctly interpret results.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Laboratory and Precision Medicine for the series “Adult Spontaneous Hypoglycaemia”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/jlpm-20-111
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm-20-111). The series “Adult Spontaneous Hypoglycaemia” was commissioned by the editorial office without any funding or sponsorship. RG is the unpaid Guest Editor of the series and serves as an unpaid editorial board member of the Journal of Laboratory and Precision Medicine from March 2020 to March 2022. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2009;94:709-28. [Crossref] [PubMed]
- Censi S, Mian C, Betterle C. Insulin autoimmune syndrome: from diagnosis to clinical management. Ann Transl Med 2018;6:335. [Crossref] [PubMed]
- Fineberg SE, Kawabata TT, Finco-Kent D, et al. Immunological responses to exogenous insulin. Endocr Rev 2007;28:625-52. [Crossref] [PubMed]
- Waldron-Lynch F, Inzucchi SE, Menard L, et al. Relapsing and remitting severe hypoglycemia due to a monoclonal anti-insulin antibody heralding a case of multiple myeloma. J Clin Endocrinol Metab 2012;97:4317-23. [Crossref] [PubMed]
- Haastrup MB, Henriksen JE, Mortz CG, et al. Insulin allergy can be successfully managed by a systematic approach. Clin Transl Allergy 2018;8:35. [Crossref] [PubMed]
- Atkinson MA, Maclaren NK, Riley WJ, et al. Are insulin autoantibodies markers for insulin-dependent diabetes mellitus? Diabetes 1986;35:894-8. [Crossref] [PubMed]
- Fineberg SE, Biegel AA, Durr KL, et al. Presence of insulin autoantibodies as regular feature of nondiabetic repertoire of immunity. Diabetes 1991;40:1187-93. [Crossref] [PubMed]
- Till AM, Kenk H, Rjasanowski I, et al. Autoantibody-defined risk for Type 1 diabetes mellitus in a general population of schoolchildren: results of the Karlsburg Type 1 Diabetes Risk Study after 18 years. Diabet Med 2015;32:1008-16. [Crossref] [PubMed]
- Wong SL, Priestman A, Holmes DT. Recurrent hypoglycemia from insulin autoimmune syndrome. J Gen Intern Med 2014;29:250-4. [Crossref] [PubMed]
- Arioglu E, Andewelt A, Diabo C, et al. Clinical course of the syndrome of autoantibodies to the insulin receptor (type B insulin resistance): a 28-year perspective. Medicine (Baltimore) 2002;81:87-100. [Crossref] [PubMed]
- Willard DL, Stevenson M, Steenkamp D. Type B insulin resistance syndrome. Curr Opin Endocrinol Diabetes Obes 2016;23:318-23. [Crossref] [PubMed]
- Martins LM, Fernandes VO, Carvalho MMD, et al. Type B insulin resistance syndrome: a systematic review. Arch Endocrinol Metab 2020;64:337-48. [Crossref] [PubMed]
- Clark PM. Assays for insulin, proinsulin(s) and C-peptide. Ann Clin Biochem 1999;36:541-64. [Crossref] [PubMed]
- Hirata Y, Nishimura H, Tominaga M, et al. Spontaneous hypoglycemia with insulin-autoimmunity. Nihon Naika Gakkai Zasshi 1972;61:1296-304. [Crossref] [PubMed]
- Takayama-Hasumi S, Eguchi Y, Sato A, et al. Insulin autoimmune syndrome is the third leading cause of spontaneous hypoglycemic attacks in Japan. Diabetes Res Clin Pract 1990;10:211-4. [Crossref] [PubMed]
- Folling I, Norman N. Hyperglycemia, hypoglycemic attacks, and production of anti-insulin antibodies without previous known immunization. Immunological and functional studies in a patient. Diabetes 1972;21:814-26. [Crossref] [PubMed]
- Virally ML, Timsit J, Chanson P, et al. Insulin autoimmune syndrome: a rare cause of hypoglycaemia not to be overlooked. Diabetes Metab 1999;25:429-31. [PubMed]
- Yukina M, Nuralieva N, Solovyev M, et al. Insulin autoimmune syndrome. Endocrinol Diabetes Metab Case Rep 2020; Epub ahead of print. [Crossref] [PubMed]
- Rajpal A, Kassem LS, Moscoso-Cordero M, et al. Clopidogrel-Induced Insulin Autoimmune Syndrome: A Newly Recognized Cause of Hypoglycemia in a Patient Without Diabetes. J Endocr Soc 2017;1:1217-23. [Crossref] [PubMed]
- Bresciani E, Bussi A, Bazzigaluppi E, et al. Insulin autoimmune syndrome induced by α-lipoic acid in a Caucasian woman: case report. Diabetes Care 2011;34:e146 [Crossref] [PubMed]
- Lohmann T, Kratzsch J, Kellner K, et al. Severe hypoglycemia due to insulin autoimmune syndrome with insulin autoantibodies crossreactive to proinsulin. Exp Clin Endocrinol Diabetes 2001;109:245-8. [Crossref] [PubMed]
- Church D, Cardoso L, Bradbury S, et al. Diagnosis of insulin autoimmune syndrome using polyethylene glycol precipitation and gel filtration chromatography with ex vivo insulin exchange. Clin Endocrinol (Oxf) 2017;86:347-53. [Crossref] [PubMed]
- Ismail AA. The insulin autoimmune syndrome (IAS) as a cause of hypoglycaemia: an update on the pathophysiology, biochemical investigations and diagnosis. Clin Chem Lab Med 2016;54:1715-24. [Crossref] [PubMed]
- Williams AJ, Bingley PJ, Bonifacio E, et al. A novel micro-assay for insulin autoantibodies. J Autoimmun 1997;10:473-8. [Crossref] [PubMed]
- Ismail AA. The double whammy of endogenous insulin antibodies in non-diabetic subjects. Clin Chem Lab Med 2008;46:153-6. [Crossref] [PubMed]
- Fahie-Wilson M, Halsall D. Polyethylene glycol precipitation: proceed with care. Ann Clin Biochem 2008;45:233-5. [Crossref] [PubMed]
- Cappellani D, Macchia E, Falorni A, et al. Insulin Autoimmune Syndrome (Hirata Disease): A Comprehensive Review Fifty Years After Its First Description. Diabetes Metab Syndr Obes 2020;13:963-78. [Crossref] [PubMed]
- Ismail AA. Testing for insulin antibodies is mandatory in the differential diagnosis of hypoglycaemia in nondiabetic subjects. Clin Endocrinol (Oxf) 2012;76:603-4. [Crossref] [PubMed]
- Flier JS, Kahn CR, Roth J, et al. Antibodies that impair insulin receptor binding in an unusual diabetic syndrome with severe insulin resistance. Science 1975;190:63-5. [Crossref] [PubMed]
- Kahn CR, Flier JS, Bar RS, et al. The syndromes of insulin resistance and acanthosis nigricans. Insulin-receptor disorders in man. N Engl J Med 1976;294:739-45. [Crossref] [PubMed]
- Malek R, Chong AY, Lupsa BC, et al. Treatment of type B insulin resistance: a novel approach to reduce insulin receptor autoantibodies. J Clin Endocrinol Metab 2010;95:3641-7. [Crossref] [PubMed]
- Taylor SI, Grunberger G, Marcus-Samuels B, et al. Hypoglycemia associated with antibodies to the insulin receptor. N Engl J Med 1982;307:1422-6. [Crossref] [PubMed]
- Yu S, Yang G, Dou J, et al. Comparison of Two Autoimmune Dysglycemia Syndromes: Insulin Autoimmune Syndrome (IAS) and Type B Insulin Resistance Syndrome (B-IRS). Horm Metab Res 2019;51:723-8. [Crossref] [PubMed]
- Kawashiri SY, Kawakami A, Fujikawa K, et al. Type B insulin resistance complicated with systemic lupus erythematosus. Intern Med 2010;49:487-90. [Crossref] [PubMed]
- Łebkowska A, Krentowska A, Adamska A, et al. Type B insulin resistance syndrome associated with connective tissue disease and psoriasis. Endocrinol Diabetes Metab Case Rep 2020; Epub ahead of print. [Crossref] [PubMed]
- Sjöholm Å, Pereira MJ, Nilsson T, et al. Type B insulin resistance syndrome in a patient with type 1 diabetes. Endocrinol Diabetes Metab Case Rep 2020; Epub ahead of print. [Crossref] [PubMed]
- Klubo-Gwiezdzinska J, Lange M, Cochran E, et al. Combined Immunosuppressive Therapy Induces Remission in Patients with Severe Type B Insulin Resistance: A Prospective Cohort Study. Diabetes Care 2018;41:2353-60. [Crossref] [PubMed]
- Supra-Regional Assay Service. Insulin Receptor Antibodies. Available online: http://www.sas-centre.org/centres/hormones/cambridge
Cite this article as: Hughes LE, Fenn JS, Ford C, Gama R. Autoimmune hypoglycaemia: a narrative review from the laboratory perspective. J Lab Precis Med 2021;6:20.


