High-density lipoprotein: a double-edged sword in cardiovascular physiology and pathophysiology
Introduction
A healthy high-density lipoprotein (HDL) in a healthy body—the Latin phrase: mens sana in corpore sano (a healthy mind in a healthy body) adopted to describe HDL function
HDL is one of the major classes of circulating lipoproteins that enable fat transport within the body. HDL was first isolated in 1929 from the horse serum by Michel Macheboeuf (1) at Pasteur Institute as a lipid-rich alpha-globulin (at that time the term “high-density lipoprotein” was not coined). In 1951, Barr et al. (2) suggested that patients who survived a coronary occlusion (acute myocardial infarction) or with other unequivocal evidence of atherosclerosis-related complications had several abnormalities in plasma proteins including a reduction in the alpha lipoprotein content. The application of analytical ultracentrifugation, developed by Gofman et al. (3) enabled the evaluation of plasma lipoproteins in clinical setting and led to the identification of reduced HDL as a risk factor for atherosclerosis (4). In 1975, Miller et al. (5) hypothesized that increased risk for coronary artery disease (CAD) is inversely related to plasma HDL level owing to the ability of HDL particle to remove cholesterol from the developing atherosclerotic lesions. Subsequent prospective epidemiological studies provided further evidence in favor of HDL-hypothesis of atherosclerosis (6-8). Thus, a meta-analysis of four large epidemiological studies by Gordon et al. (9) estimated that for each one mg/dL increase in the HDL-cholesterol, the risk for CAD was reduced by 2–3%. However, recent research involving therapies that rise HDL concentration, genetic human studies or genetic animal models that alter HDL metabolism and lead to changes in HDL concentration did not show a change in the cardiovascular risk, atherosclerotic plaque load or incidence of CAD-related events commensurate with the HDL change. The results of these studies dealt a blow to the HDL-hypothesis of atherosclerosis and CAD and ended the era of HDL-rising therapies as a strategy to prevent atherosclerosis and CAD. In recent years, there has been a paradigm shift in the investigation of HDL as a therapeutic target, from the measurement of HDL concentration to the assessment of HDL function [i.e., reverse cholesterol transport (RCT)] as a response to various therapeutic interventions (10,11). Furthermore, mounting evidence suggests that cardiovascular risk factors and various morbid conditions alter the HDL composition and properties transforming this lipoprotein from a healthy (functional) particle with vasoprotective and anti-atherosclerotic properties into a dysfunctional and pro-atherosclerotic particle (12). The aim of this review was to summarize the current knowledge on the role of HDL in cardiovascular physiology and pathophysiology of cardiovascular disease (CVD) and the structural and functional alterations that diminish the physiological role of HDL and transform this lipoprotein into a dysfunctional pro-atherosclerotic particle. The involvement of HDL in other diseases and inborn errors affecting HDL content or metabolism are not covered.
HDL structure
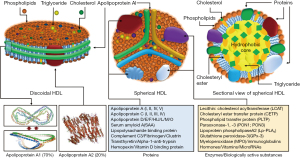
HDLs are the smallest (5–17 nm diameter), but densest (1.063–1.25 g/mL) class of plasma lipoproteins. Structurally, HDL particle represents a protein-lipid supramolecular complex, with both these components varying markedly in type and quantity. HDLs are highly complex structures that undergo dynamic changes in shape, size and composition through continuous interactions with various enzymes and cellular receptors across various tissues throughout their life cycle. The complete set of proteins and lipids in the structure of HDL are termed as HDL proteome and lipidome, respectively. A recent study has compiled a list of 566 different proteins that were found associated with HDL in 37 different studies (13). However, apolipoprotein A1 (Apo-A1), and apolipoprotein A2 (Apo-AII) were reported in all studies and these 2 proteins comprise 90% of HDL protein mass (13,14). In 75% of studies, only 21 proteins were found in the structure of HDL (13). Proteins are major structural and functional components of HDL. HDL proteins include several major subclasses such as, apolipoproteins, enzymes, lipid transfer proteins, acute-phase response proteins, complement system components, proteinase inhibitors and proteins with other biological functions (12,15). The lipid fraction of HDL contains nearly all classes of lipids including, glycerophospholipids, cholesterol, cholesteryl esters, triglycerides, diacylglycerols and sphingolipids (e.g., sphingosine-1-phosphate). Lipids are not inert structural constituents of HDL or simply intended for transport through the plasma, but they have an important biological activity, as well (16). HDL is protein-rich and the protein-to-lipid ratio varies from 10:1 in pre-β-HDL to 1:2 in large HDL2 (14). HDL has a remarkable absorptive capacity. Thus, many constituents of plasma are found in the structure of HDL in concentrations by one or two orders of magnitude higher than in plasma (10). Structural characteristics of HDL are shown in Figure 1. Although, HDL are heterogeneous and differ markedly in size and shape, probably reflecting different metabolic stages of the particle, the most commonly HDL forms found in plasma are discoidal (nascent) and spherical (mature) HDL. Discoidal HDL represents a phospholipid bilayer similar to plasma membrane consisting of 100 to 150 phospholipid molecules and 2 to 5 molecules of Apo-A1 forming a belt around the lipid moiety. Discoidal HDL has a thickness of 20 Å and a diameter of 90 to 100 Å (10,17,18). Discoidal HDL comprises approximately 10% of overall circulating HDL. Apo-A1 and Apo-AII are the main protein components of discoidal HDL whereas lipid components consist of phospholipids (mainly) and few molecules of free cholesterol, cholesteryl esters and triglycerides. Discoidal HDL are transformed gradually into spherical HDL, which is the major type of HDL in circulation. Spherical HDL consists of a hydrophobic center containing cholesteryl esters and to a lesser degree triglyceride surrounded by a protein-phospholipid monolayer shell (Figure 1). Each mature spherical HDL contains 50–130 phospholipid molecules (phosphatidylcholine, sphingolipids, isoprenoids and acylglycerols), 30–90 molecules of cholesteryl esters, 10–50 molecules of free cholesterol, 10–20 molecules of triglycerides and approximately 80 proteins (19). Expressed as a percentage, 35% to 50% of HDL lipids is comprised of phospholipids, 30% to 40% of cholesteryl esters, 5% to 12% of triglycerides, 5% to 10% of free cholesterol and 5% to 10% of sphingolipids (20). Apart from proteins and lipids HDL particles contain many other substances with biological activity including hormones, vitamins and microRNAs (miRs) (21). A number of miRs including miR-24, miR-33a, miR-30c, miR-92a, miR-122, miR-125a, miR-126, miR-145, miR-146a, miR-150, miR-155, miR-223, miR-378, miR-486, and miR-17/92 cluster have been identified in the HDL particles suggesting that HDL is an effective carrier of circulating functional miRs to target cells (21-24). miRs may have a role in the stabilization of HDL structure, as well (25). Immune cells, pancreatic beta cells, neurons and neutrophils appear to export miRNAs to HDL, which delivers them to target cells like hepatocytes, endothelial and microglial cells (26). In analogy with proteome and lipidome, HDL miRs cargo is not static and changes in physiological and pathological conditions. HDL are classified into different classes depending on the analytical method used to characterize them. HDL is highly heterogeneous consisting of several subclasses according to density, size, shape and lipid and protein composition. Most commonly used HDL classifications are summarized in Table 1 (15,27). HDL circulates in plasma for 2 to 4 days (11).
Table 1
| Technique/HDL subclass | Units |
|---|---|
| Density (ultracentrifugation) | Gram/deciliter |
| HDL2 | 1.063–1.125 |
| HDL3 | 1.125–1.21 |
| Size (Electrophoresis) | Nanometer |
| HDL2b | 9.7–12.0 |
| HDL2a | 8.8–9.7 |
| HDL3a | 8.2–8.8 |
| HDL3b | 7.8–8.2 |
| HDL3c | 7.2–7.8 |
| Size plus particle number (nuclear magnetic resonance) | Nanometer |
| Large HDL | 8.8–13.0 |
| Medium HDL | 8.2–8.8 |
| Small HDL | 7.3–8.2 |
| Charge plus size (two-dimensional electrophoresis) | Particle |
| Pre-β-HDL | preβ1 and preβ2 |
| α-HDL | α1, α2, α3 and α4 |
| Pre-α-HDL | preα1, preα2, preα3 |
| Shape and size (agarose gel) | Shape |
| α-HDL | Spherical |
| Preβ-HDL | Discoidal |
| Protein composition (Antibody-based Electroimmunodiffusion) | Apolipoprotein |
| LpA-I* | Apo-AI |
| LpA-I:A-II | Apo-A1 plus ApoA-II |
*HDL can be separated into particles containing apoA-I and ApoA-II (referred as LpA-I:A-II) or without apoA-II (referred as LpA-I). Apo, apolipoprotein; HDL, high density protein.
Apo-A1 is the principal protein component and the backbone of HDL (28). Apo-A1 is a fascinating and multitasking protein that participates in almost all known biological functions of HDL. Apo-A1 is a 28-kD polypeptide with 243 amino acid residues. Apo-A1 is produced as a preproapolipoprotein A1 with an extra 24-amino acid N-terminal sequence which is removed after proteolytic cleavage (29). The largest part of secondary structure of Apo-A1 consists of amphipathic alpha-helices with apolar amino acid residues located in one side of the alpha-helix (the side facing lipid components) and polar residues (i.e., lysine and arginine amino acids) oriented on the other side of the alpha-helix (the side that enables aqueous actions such as interaction with receptors or enzymes). The alpha-helices are separated by proline residues (an amino acid that does not form alpha helix) which serve as hinges giving the molecule flexibility allowing large conformational changes (molecular spatial adaptations) of Apo-A1 that take place during HDL maturation (transition from discoidal to spherical HDL) (30,31). Although several molecular models of spatial conformation of HDL have been proposed, the belt (in discoidal HDL) and trefoil (in spherical HDL) models are mostly accepted (15). Apo-A1 conformational adaptation enables the interaction of HDL with various receptors such as, ATP binding cassette transporters A1 (ABCA1) and G1 (ABCG1), scavenger receptor B1 (SR-B1) and various enzymes such as, lecithin:cholesterol acyltransferase (LCAT) (31). Approximately 90% of Apo-A1 circulates bound to HDL whereas 10% circulates as lipid-free Apo-A1 at a concentration of 90–250 mg/dL with a slightly higher concentration in women than in men (31). In pathophysiological conditions characterized by heightened inflammatory state, the content of Apo-A1 in the HDL is lowered owing to 2 potential factors, displacement of the protein by acute-phase reactants such as serum amyloid A (SAA) (32) or cytokine-related concomitant increase in the expression of SAA and inhibition of expression of apo-A1 and paraoxonase 1 (PON1) (33). It appears that specific molecular domains of Apo-A1 carry out specific functions such as, interaction with receptors or enzyme activation (i.e., LCAT) (31). Other proteins and enzymes associated with HDL are shown in Figure 1.
HDL metabolism
The main aspects of HDL metabolism are summarized in Figure 2. The initial step in the HDL metabolism is the synthesis of Apo-A1, which is secreted by hepatocytes (approximately 80% of de novo synthesis) or intestinal mucosa (20%). In the classical model, Apo-A1 is secreted either as free protein or associated with few phospholipids as lipid-poor protein (10). These precursor small discoidal protein-phospholipid particles are called pre-β-1 HDL (nascent HDL) because of its migration on two-dimensional gel electrophoresis. Other sources of Apo-A1 (and/or pre-β-1 HDL) are catabolism of mature HDL and lypolyzed apolipoprotein B lipoproteins. Once in circulation apo-A1 or pre-β-1 HDL interact with ABCA1 transporters, which represent cholesterol-phospholipid transporters expressed in hepatocytes, enterocytes, macrophages and other tissues. These receptors allow flowing of free cholesterol and phospholipids (lipid efflux) from various tissues to apo-A1 or pre-β-1 HDL gradually transforming these initial particles into discoidal HDL (or α4-HDL). Other cellular receptors involved in the lipid efflux from the cells (tissues) are ABCG1 and SR-B1. On the surface of discoidal HDL, free cholesterol and phospholipids serve as substrates for the enzyme LCAT (EC2.3.1.43), first described by Glomset in 1962 (34). The enzyme converts free cholesterol into cholesteryl ester and phospholipids into lysophospholipids (e.g., lysophosphatidylcholine in case the substrate was phosphatidylcholine). Approximately 75% of plasma LCAT is associated with HDL, but the enzyme can generate cholesteryl esters in apoprotein-B containing lipoproteins, as well (35). The Apo-A1 serves as an activator of LCAT. Cholesteryl esters penetrate discoidal HDL phospholipid monolayer and accumulate together with a fewer amount of triglycerides in the hydrophobic center of the particle. This spatial shift of cholesteryl esters from the surface to the inside of discoidal HDL has at least 2 consequences: first, the sequestration of cholesteryl esters in the center of the structure reduces the content of cholesterol on the surface of discoidal HDL preventing the LCAT reaction from reaching the equilibrium (forcing the reaction to become unidirectional) and allowing continuation of cholesterol efflux from the tissues and esterification by LCAT, and second, gradual accumulation of cholesteryl ester and triglycerides transforms discoidal HDL into a spherical structure, which represents the predominant form of HDL in plasma. Size expansion and shape change are associated with a change in the Apo-A1 spatial conformation from a belt-like to a trefoil-like structure. In the latter conformation 4 to 5 Apo-A1 molecules are arranged to form a trefoil-like structure (36). Conformation changes of Apo-A1 are crucial for HDL maturation and functions including RCT.
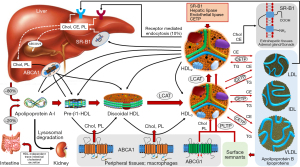
The newly formed spherical HDL (or HDL3) is matured further by continuation of the LCAT reaction (and consequently cholesterol efflux via interaction with ABCA1 and ABCG1 receptors) and the action of lipid transfer proteins. Two lipid transfer proteins—the cholesteryl ester transfer protein (CETP) and the phospholipid transfer protein (PLTP) —are mostly responsible for further maturation of spherical HDL. Both proteins regulate the composition and the size of HDL in circulation and play important roles in controlling plasma levels of HDL.
CETP is a hydrophobic glycoprotein that is secreted mainly from the liver and that circulates in plasma, bound mainly to HDL (37). CETP promotes the transfer of cholesteryl esters from HDL to all classes of apolipoprotein B-containing lipoproteins in exchange with triglycerides. Most of the cholesteryl esters transferred from HDL originate in the LCAT reaction whereas triglycerides transferred to HDL originate predominantly from triglyceride-rich apolipoprotein B-containing lipoproteins (chylomicrons and very low-density lipoproteins) (38). The PLTP is a 476 amino acid residue glycoprotein with a molecular weight of 53 kDa (mature protein without signal peptide). However, the molecular weight has been reported up to 80 kDa depending on the degree of glycosylation (39). The key role of PLTP in HDL metabolism consists in the transport of phospholipids released at the time of lipolysis of chylomicrons and very-low density lipoproteins by lipoprotein lipase. The PLTP enables transfer of excess surface phospholipids from triglyceride-rich lipoproteins to HDL facilitating the formation of smaller lipoprotein remnants and maturation of HDL particles (40,41). As a result of the action of CETP and PLTP, the initially small HDL3 particles increase in size and their composition is altered (enriched in triglycerides and depleted in cholesteryl esters). These particles are called HDL2 (Figure 2).
Catabolism of HDL differs markedly compared with apolipoprotein B-containing lipoproteins. In distinction to low-density lipoprotein (LDL), only a minor proportion of HDL (approximately 10%) is eliminated by the cells as holoparticle. In this poorly understood pathway, the Apo-A1 interacts with ectopic F0F1-ATPase complex (the mitochondrial F0F1-ATPase is activated by protons to produce ATP) leading to formation of adenosine diphosphate (ADP), which activates purinergic receptors enabling HDL uptake by an unidentified low-affinity receptor (10). Other clearance pathways may include micropinocytosis in cells of lymphatic system (42), clathrin-coated pits in endothelial cells (43), transcytosis in polarized cells (44), which allows the particle to come in contact with cholesterol-loaded macrophages in the subendothelial space and fusion and incorporation of HDL within the cellular plasma membranes (45). However, the predominant way of HDL catabolism is disintegration of the particle after interaction with enzymes and cellular receptors. Thus, HDL2 continues to interact with apoprotein B-containing lipoproteins exchanging cholesteryl esters with triglycerides via CETP. Furthermore, HDL2 interacts with SR-B1 receptors supplying cholesteryl esters and phospholipids to hepatocytes and endocrine organs involved in the synthesis of steroid hormones (adrenal gland and gonads). Finally, HDL2 particles undergo lipolysis (hydrolysis of triglycerides) by hepatic lipase and endothelial lipase. The concerted action of CETP, transfer proteins and SR-B1 receptors transform HDL2 particles into smaller particles including precursor HDL3 and pre-β-1 HDL or even free Apo-A1 giving rise to multiple cycles of transformation between the HDL particles (46,47). The HDL particles produced during HDL catabolism follow the same way as de novo produced precursor particles. The released Apo-A1 (either as free protein or lipid-poor particle) interacts with ABCA1 receptors and re-enters the pathway of HDL synthesis (and maturation) in analogy with the de novo synthesized Apo-A1. A portion of circulating Apo-A1 is filtered by renal glomeruli. The filtered Apo-A1 interacts with cubilin/megalin recepters in the proximal tubular cells, is internalized within these cells and undergoes lysosomal degradation (48). According to one recently proposed model, all HDL subfractions are secreted by the liver into plasma and circulate within the secreted size (i.e., not changing in size) for 1 to 4 days before they are cleared from the circulation (49).
HDL functions
HDL performs multiple physiological functions. Outside the role in lipid transport and metabolism, HDL plays multiple other actions (so-called pleiotropic actions) mostly related to various proteins and enzymes associated with this structure. In this regard, HDL may be seen as a circulating vehicle loaded with multiple biological activities. Main functions of HDL are summarized in Figure 3.
RCT from peripheral tissues to the liver is the best known function of HDL, which has been seen for decades as synonymous with the anti-atherosclerotic properties of this class of lipoproteins. The concept that HDL serves as a shuttle removing excess cholesterol from the lipid-laden macrophages in peripheral tissues to the liver was first proposed by Glomset (50) in 1968. Thus, when circulating HDL comes into contact with the cell surface (which has a normal cholesterol content), the cholesterol concentration gradient favors passive aqueous diffusion of free cholesterol from the cell to the HDL (51). At higher cholesterol levels, the non-aqueous cholesterol efflux from cells to HDL is facilitated by tethering of HDL to SR-BI and ABCA1 receptors (47). Although, nearly all HDL-associated apolipoproteins recognize ABCA1 receptors and may obtain cholesterol and phospholipids from them, Apo-A1 shows the most avid interaction with these receptors and is responsible for the majority of cholesterol efflux via ABCA1 receptors (52). Interestingly, when cholesterol reaches a high concentration in hepatocytes, a portion of cholesterol is oxidized to oxysterols activating nuclear liver X receptors α and β inducing gene transcription and mRNA synthesis for ABCA1 and ABCG1 receptors, which facilitate the cholesterol efflux (53). However, the regulation of ABCA1 receptor expression is complex and poorly understood. It appears to be under the control of miRs with miR33, miR122 and miR144 reducing the ABCA1 synthesis (10). The key role of ABCA1 receptors in promoting cholesterol efflux from tissues to Apo-A1 (or other HDL particles) has been demonstrated in the ABCA1-gene-knockout mouse model (51) or patients with Tangier’s disease (54) (mutations in the ABCA1 gene), with both conditions associated with the inability to produce HDL. As stated earlier in this review, as a result of LCAT reaction, the content of free cholesterol on the surface of HDL is kept low due to cholesterol esterification and migration (and trapping) of cholesteryl esters inside the particle. Following esterification by LCAT enzyme, cholesteryl esters from HDL particles are returned to the liver predominantly via two mechanisms: transfer of cholesteryl esters to apolipoprotein B-containing lipoproteins in exchange with triglycerides with participation of CETP and PLTP (followed by receptor-mediated LDL uptake and internalization by hepatocytes) and via selective removal by SR-B1 receptors in hepatocytes (Figure 2). Cholesteryl esters in the liver are used for synthesis of biliary acids, which are excreted in bile or re-transferred to circulating Apo-A1 or nascent HDL via ABCA1 receptors. Apart from the role in cholesterol homeostasis, RCT is involved in multiple other physiological processes seemingly not related to lipid metabolism (10). Although, the HDL(Apo-A1)-ABCA1 axis (or the Glomset model) plays the key role in the cholesterol efflux and RCT, mice deficient in ABCA1 or Apo-A1 appear to have no disturbances in cholesterol homeostasis (55). This evidence suggests the existence of a macrophage-specific RCT (56,57) in mice, which is distinct from the above-described RCT model. Whether, a macrophage-specific RCT exists in humans remains unknown. Finally, there is evidence to suggest the existence of HDL-independent RCT pathway or so-called trans-intestinal cholesterol excretion (58).
HDL is a major regulator of cholesterol content in the cells and of the cholesterol homeostasis, in general. Cholesterol metabolism is complex but in general, cells cannot metabolize it into smaller units to extract energy as they do with fatty acids—a metabolic feature that predispose cells to cholesterol overload. As explained earlier, HDL interacts with cellular cholesterol transporters enabling cholesterol efflux from the cells and cellular cholesterol unloading. This keeps cellular cholesterol pool in the cells within the physiological limits and prevents cholesterol overloading. Once incorporated in the HDL particles, cholesterol is redistributed to other lipoprotein classes, tissues and finally to the liver where cholesterol is targeted for final elimination.
Although HDL is seen as a tool to remove cholesterol from the cells, depending on the metabolic needs, this particle may supply cholesterol to the tissues. This function is accomplished by interactions of HDL with SR-B1 receptors in liver and endocrine organs producing steroid hormones (adrenal gland, ovary and testicular Leydig cells) which are in need of a constant supply of cholesterol for steroidogenesis (59). Although these cells have the molecular machinery to produce cholesterol, cholesterol synthesis is highly complex needing approximately 200 enzymatic activities and is expensive in terms of energetic expenditure. For these reasons, cells involved in steroidogenesis prefer external cholesterol supply by plasma lipoproteins including HDL.
One of the most important functions of HDL is its ability to interfere with nitric oxide (NO) metabolism through which it exerts profound effects on endothelial and vascular function. HDL activates endothelial nitric oxide synthase (eNOS) via interaction of Apo-A1 with SR-B1 receptors in endothelial cell caveolaes and via sphingosine-a-phosphate (S1P) signaling pathway initiated by interaction of S1P with S1P receptors in the membranes of endothelial cells promoting eNOS activation (60-62). HDL from healthy subjects and Apo-A1 stimulate NO release from human umbilical vein or human aortic endothelial cells and upregulate expression of eNOS through multisite phosphorilation in a downstream signaling cascade (63,64). Apart from Apo-A1, other HDL associated enzymes and apolipoproteins such PON1 (65) and apolipoprotein E (isoforms E3 and E2 but not E4) (66) stimulate eNOS and increase NO production. The interaction of HDL with SR-B1 receptors stimulates migration and proliferation of endothelial cells promoting re-endothelialization and preservation of integrity of vascular endothelium, independent of eNOS activation and NO signaling (67). Experiments in rats have shown that HDL and Apo-A1 promote proliferation and migration of endothelial progenitor cells and angiogenesis via phosphatidylinositol-3-kinase/Akt-dependent cyclin D1 activation (68). Considering multiple biological actions of NO, many effects of HDL on endothelium including anti-inflammatory, antioxidant and anti-apoptotic effects, cell proliferation, migration, endothelial repair, angiogenesis and improved cell survival, inhibition of cell adhesion molecules and antithrombotic action and inhibition of blood coagulation are exerted through HDL-induced increased NO availability in endothelium (19,62). In aggregate, these data demonstrate the key role of HDL in maintaining endothelial function, vascular health and anti-atherosclerotic action.
HDL exerts anti-inflammatory actions mostly related to Apo-A1 and other proteome (PON1 or clustrin) or lipidome (sphingosine-1-phosphate) constituents. Initial signals linking HDL with inflammation suppression came from demonstration of elevated C-reactive protein levels in patients with hypoalphalipoproteinemia—a genetic condition characterized by very low HDL cholesterol (69). Apo-1 has been demonstrated to inhibit cytokine-induced expression of cellular adhesion molecules such as, vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1) (70) and E-selection and shear-stress induced monocyte expression of integrin CD11b (71). The inhibition of expression of adhesion molecules in endothelial cells decreases trans-endothelial migration and recruitment of immunocompetent cells (blood monocytes) from circulation to the vascular arterial wall (72), considered to represent initial events in atherosclerotic lesion formation. HDL inhibits LDL-induced monocyte chemoattractant protein-1 (MCP-1) (73)—a key chemokine that regulates migration and infiltration of monocytes/macrophages to the arterial wall. It has demonstrated that Apo-A1 inhibits activated T cell-monocyte/macrophage contact-mediated stimulation of production of interleukin 1β and tumor necrosis factor alpha (74). Furthermore, HDL inhibits NF-kB activity and by this action, it suppresses the activation of monocytes and endothelial cells (75). The HDL ability to curb inflammation mediated by monocytes, macrophages and neutrophils appears to be another key mechanism through which HDL exerts anti-atherosclerotic and cardiovascular protection.
Another important function of HDL is its antioxidant action through which HDL prevents lipid oxidation and removes oxidized products. HDL proteome contains a number of antioxidant enzymes such as PON1, lipoprotein-associated phospholipase A2 (called platelet-activating factor acetylhydrolase) and LCAT (76). PON1 has been the most studied enzyme with antioxidant action. Experimental studies have shown that PON1 protects HDL and LDL from oxidation (77,78) whereas mice lacking serum PON1 (PON1 knockout mice) loose this protection and are susceptible to organophosphate toxicity and atherosclerosis (79). The presence of Apo-A1 appears to be crucial for PON1 activity (12). Both LCAT and lipoprotein-associated phospholipase A2 exert antioxidant action by hydrolyzing oxidized acyl moieties from oxidized phospholipids (80). Apo-A1 possesses also important antioxidant properties by binding and removing of peroxidation products from LDL, cholesteryl esters and phosphatidylcholine (81). One of the most important antioxidant actions of HDL is prevention of LDL by lipid peroxidation products produced by macrophages (82) and an inverse correlation between circulating HDL level and oxidized LDL concentration has been demonstrated (83). Small, dense HDL3 particles appear to have a greater capacity to prevent LDL oxidation than larger and lighter HDL2 (80). HDL reduces tumor necrosis factor alpha-induced superoxide production in endothelial cells possibly mediated by inhibitory effect of HDL-associated lysosphingolipids on NADPH oxidase following interaction with sphingosine-1-phosphate receptor 3 (S1P3) and SR-B1 receptors (12,84). HDL binds lipid peroxidation products and HDL-bound hydroperoxides and hydroxides are transferred to liver after interaction of HDL with SR-B1 receptors where they are eliminated by hepatocytes (19).
HDL has immunomodulatory properties and participates in host defense by affecting innate and adaptive immune responses (85). The immunomodulatory properties of HDL have been investigated in the setting of autoimmune diseases but implications for CVD remain unknown. One of the earliest evidence linking HDL with innate immunity is HDL-induced reduction of lipopolysaccharide (LPS) toxicity (86), later found to be attributed to sequestration of LPS by HDL which prevents transduction of proinflammatory pathway initiated by LPS-Toll-like receptor 4 (TLR4)/CD14 complex (LPS receptor) (87). TLR4 is expressed in innate immune cells including monocytes, macrophages and dendritic cells and is activated by LPS. TLR4 triggers the MyD88-dependent signaling pathway inducing production of pro-inflammatory cytokines and cell activation. Furthermore, HDL induces the expression and activation of activating transcription factor 3, a transcriptional modulator that inhibits TLR signaling (88). It has been suggested that HDL modulates innate or adaptive immunity by altering cholesterol content in the lipid rafts—cell membrane segments with a higher cholesterol content that harbor many membrane receptors in B and T lymphocytes including TLR (85). Thus, by depleting cholesterol from lipid rafts, HDL inhibits TLR trafficking mediated by MyD88 (89), the ability of antigen-presenting cells (macrophages and dendritic cells) to activate T cells (90) and block conversion of monocytes into migratory dendritic cells via enzyme platelet-activating factor acetylhydrolase (91). HDL constituents including Apo-L1, sphingosine-1-phosphate, immunoglobulins and components of complement system participate directly in the immune response. HDL role in the regulation of immune response and implications for atherosclerosis have been recently reviewed (92). In one recent population-based cohort study, HDL showed a U-shaped relationship with the risk for infectious diseases with higher risk of developing infection in subjects with low and high HDL level (93).
HDL exerts many other biological actions. The cytoprotective actions of HDL on endothelial cells may involve inhibition of apoptosis in these cells. Thus, HDL prevents apoptosis of human umbilical venous endothelial cells induced by tumor necrosis factor-alpha via inhibition of cysteine protease P32-like protease activity (94). HDL from healthy subjects induced expression of the endothelial antiapoptotic Bcl-xL protein and reduced endothelial cell apoptosis in vitro and in apolipoprotein E-deficient mice in vivo (95). HDL exerts an antithrombotic/anticoagulant action. Apart from the inhibition of expression of adhesion molecules, HDL enhances prostacyclin synthesis, attenuates expression of tissue factor and E-selectin, enhances inactivation of coagulation factor V, upregulates tissue plasminogen activator enhancing fibrinolysis, downregulates thrombin generation through protein C pathway and attenuates platelet activation (96,97). Acute infusion of reconstituted HDL reduces platelet aggregation and is highly effective at inhibiting the heightened platelet reactivity in diabetic patients, partly through reducing the cholesterol content in platelet membranes (98). HDL modulates glucose metabolism via actions on organs like pancreas, skeletal muscle, heart, adipose tissue, liver and brain by influencing insulin secretion from pancreatic beta cells, insulin-independent glucose uptake from tissues and insulin sensitivity (99). HDL improves beta-cell function and insulin secretion through prevention cholesterol accumulation via interaction with ABCA1 and ABCG1 receptors in these cells (100) and protecting beta-cells from apoptosis (101). HDL and Apo-A1 improve insulin sensitivity and glucose uptake in skeletal muscle via activation of glycogen synthase kinase-3 and adenosine monophosphate-activated protein kinase (AMPK). Furthermore, they increase glucose uptake through increased insulin-mediated activation of phosphoinositide 3-kinase (PI3K)/Akt (protein kinase B) pathway resulting in increased GLUT4 translocation to the cell surface (102). Insulin receptor resides in the lipid raft membrane domains and HDL may interfere with insulin signaling via its impact on lipid rafts. HDL exerts an anti-ischemic and protects myocardium during ischemia-reperfusion cycles via multiple mechanisms including inhibition of inflammation, recruitment of inflammatory cells, increased NO availability and activation of salvage pathways. In a mouse model of myocardial ischemia/reperfusion, HDL and sphingosine-1-phosphate reduced infarction size by approximately 20% and 40%, respectively through inhibition of inflammatory neutrophil recruitment and cardiomyocyte apoptosis in the infarcted area (103). HDL and sphingosine-1-phosphate-mediated cardioprotection was dependent on NO and the sphingosine-1-phosphate receptor 3 (S1P3) lysophospholipid receptor, because it was abolished by pharmacological NOS inhibition and was completely absent in S1P3-deficient mice (103). By serving as an effective carrier of miRs, HDL modulates multiple cellular processes and pathways via these small non-coding RNA molecules. HDL delivered functional miRs from macrophages to endothelial cells, regulating miR target expression and cellular processes such as adhesion molecule expression and angiogenesis contributing to cellular communication within the atherosclerotic lesions (26). It has been suggested that miR-233-3p suppresses endothelial cell activation and adhesion molecule expression conferring anti-inflammatory and antiatherogenic actions (104). On the other hand, miR-24 appears to be atherogenic (105).
HDL and atherosclerotic cardiovascular disease—epidemiological evidence and HDL interventions
Following initial reports that patients presenting with acute myocardial infarction had lower alpha lipoprotein levels (2), several epidemiological population-based studies showed an inverse association between HDL and incident CVD. Several studies published in 1977 showed an association between HDL and CAD prevalence or incidence. The Cooperative Lipoprotein Phenotyping Study investigated the association between HDL and CAD prevalence in 6,859 subjects of diverse ethnicities aged 40 years or older. In each major study group, HDL was significantly higher among subjects without CAD albeit the difference was small (3–4 mg/dL). The inverse association between HDL and CAD prevalence persisted after adjustment for total cholesterol, LDL and triglycerides (6). The Framingham study investigated the association between lipid and lipoprotein values and incidence of CAD in 2,815 men and women, 49 to 82 years of age. In approximately 4 years of follow-up 142 subjects (79 males, 63 females) developed CAD with HDL showing an inverse association with incident CAD in both sexes (106). Along the same lines, the Tromso Heart Study—a prospective case-control study in Norway that included 6,595 subjects 20 to 49 years identified HDL as the best correlate of the risk for CAD over 2 years of follow-up (7). A 12-year follow-up of 2,748 participants (aged 50 to 79 years) recruited in the Framingham Heart Study showed an association between low HDL and increased risk for mortality. For men, the relative risk of being in the first HDL quintile (HDL <35 mg/dL) versus being in the top quintile (HDL >54 mg/dL), was 1.9 for all-cause mortality and 3.6 and 4.1 for death due to CVD and CAD, respectively after adjustment for standard CVD risk factors. In women, the corresponding relative risks comparing the first (HDL <45 mg/dL) versus top (HDL >69 mg/dL) quintile for all-cause, CVD and CAD mortality were 1.5, 1.6, and 3.1, respectively (107). A post-hoc analysis of the Treating to New Targets study assessed the predictive value of HDL in 9,770 patients with established CAD on statin therapy. HDL cholesterol level in patients receiving statins was predictive of the 5-year risk for major adverse cardiovascular events. After adjustment, a HDL-cholesterol >55 mg/dL was associated with 25% lower risk of cardiovascular mortality compared with subjects with a HDL cholesterol <38 mg/dL [hazard ratio of 0.75, 95% confidence interval (CI), 0.60 to 0.95]. Of note, even among patients with a LDL cholesterol below 70 mg/dL, subjects in the highest HDL quintile had a lower cardiovascular mortality compared with those in the lowest HDL quintile (108). Finally, the large Emerging Risk Factors Collaboration Study that included 302,430 subjects without a history of CAD offered strong support for an inverse association between HDL and the risk for CAD. The study reported a HR of 0.71 (95% CI, 0.68 to 0.75) between HDL cholesterol and the risk of CAD after adjusting for nonlipid risk factors and a HR of 0.78 (95% CI, 0.74 to 0.82) after further adjustment for non-HDL cholesterol, over 2.79 million person-years of follow-up (109).
Overall, the results of these studies supported the HDL-hypothesis of atherosclerosis and CAD and ushered in a new era of studies focusing on HDL-raising therapies. Numerous HDL-raising therapies and agents including fibrates, thiazolidinediones, novel peroxisome proliferator-activated receptors, niacin, statins and apabetalone were tested in clinical setting. These agents increased the HDL level between 5% and 30% (110). The results of these studies were clinically inconsistent in terms of the association between HDL increase and the clinical benefit. A 2009 meta-analysis that included 108 randomized studies involving 299,310 participants showed that there was no association between treatment-induced change in the HDL cholesterol and the risk for CAD mortality, CAD events or all-cause mortality in all analyses that adjusted for changes in LDL cholesterol. With all trials included, the change in HDL cholesterol explained almost no variability (<1%) in any of the outcomes. The meta-analysis concluded that simply increasing the amount of circulating HDL cholesterol does not reduce the risk of CAD events, CAD deaths, or all-cause mortality and that LDL cholesterol but not HDL cholesterol should be defined as the primary goal for lipid modifying interventions (111). Although some agents like niacin showed clinical benefit in interventions before statin era, no benefit was reported in niacin-statin combinations. The AIM-HIGH trial randomized 3,414 patients with atherosclerotic CVD to receive extended-release niacin or placebo on top of simvastatin and ezetimide (if needed to keep LDL below 80 mg/dL). At 2 years niacin increased the level of HDL (1.08 mmol/liter), lowered trigiyceride level (1.85 mmol/liter) and LDL cholesterol (1.91 mmol/liter). The primary outcome—a composite of death from CAD, nonfatal myocardial infarction, ischemic stroke, hospitalization for an acute coronary syndrome, or symptom-driven coronary or cerebral revascularization—occurred in 16.4% of patients in the niacin group and 16.2% of patients in the placebo group (P=0.79) over a 36-month follow-up period. The study concluded that there is no benefit of adding niacin to statin therapy despite significant improvements in the HDL and triglyceride levels (112). In the HPS2-THRIVE Collaborative Group study, 25,673 adults with vascular disease were randomized to receive 2 g of extended-release niacin plus 40 mg of laropiprant (a prostaglandin D2 receptor DP1 inhibitor used to reduce niacin-induced flushing) or placebo on top of statin therapy. Over a 3.9 years of follow-up, the primary outcome (first major vascular event: nonfatal myocardial infarction, death from coronary causes, stroke, or arterial revascularization) occurred in 13.2% of patients assigned to niacin-laropiprant combination and 13.7% of patients in the placebo group (P=0.29). Although HDL level was on average 6 mg/dL higher in the niacin-laropiprant group, the risk for major vascular events was not reduced but the risk for serious adverse events was increased compared with placebo (113). These two studies did not support the use of niacin on top of statin therapy in high-risk patients with atherosclerotic vascular disease and controlled LDL level.
The development and clinical use of CETP inhibitors nurtured hope that the powerful HDL-raising effects of these agents will translate into clinical benefit. Thus, the CETP inhibitor anacetrapib increased the HDL by 138.1% compared with pre-treatment level (114). Four randomized studies published in 2007 assessed the efficacy and safety of CETP inhibitor torcetrapib plus atorvastatin versus atorvastatin plus placebo in high-risk patients with atherosclerotic CVD. In the study by Barter et al. (115) torcetrapib was associated with a 72% increase in the HDL level but it increased cardiovascular mortality by 25%, as well. In the study by Nissen et al. (116) torcetrapib was associated with a 61% increase in the HDL level but it increased blood pressure and incidence of hypertensive cardiovascular events without a significant difference in progression of atherosclerosis, assessed by intravascular ultrasound. In the study by Kastelein et al. (117), 850 patients with heterozygous familial hypercholesterolemia were randomized to atorvastatin monotherapy or atorvastatin combined with 60 mg of torcetrapib for 2 years. The study reported that the torcetrapib/atorvastatin combination versus atorvastatin monotherapy did not reduce the progression of atherosclerosis, as assessed by a combined measure of carotid arterial-wall thickness, and was associated with progression of disease in the common carotid segment. In the study by Bots et al. (118), 752 patients with mixed dyslipidemia were randomized to receive 60 mg of torcetrapib or placebo on top of atorvastatin therapy. Carotid ultrasound imaging was performed at 6-month intervals for 24 months. Although the torcetrapib/atrovastation combination was associated with a 63.4% relative increase in the HDL and a 17.7% relative decrease in the LDL cholesterol levels, it increased systolic blood pressure and did not affect the yearly rate of change in the maximum intima-media thickness measured in 12 carotid segments. These studies showed that CETP inhibitor torcetrapib failed to bring clinical benefit in patients with atherosclerotic CVD and even increased CVD risk although it markedly increased the HDL level.
Although, the results with torcetrapib were disappointing in terms of lack of efficacy and poor safety profile, the research went on with other CETP inhibitors. Two studies assessed the safety profile of CETP inhibitor dalcetrapib. In one study, dalcetrapib was not associated with pathological effects on vascular wall over 24 months (119). In the other study that included patients at high risk for CAD, dalcetrapib reduced CETP activity, increased HDL level without affecting NO-dependent endothelial function, blood pressure or markers of inflammation or oxidative stress (120). The drug was safe and well tolerated. In the large dal-OUTCOMES trial that included 15,871 patients with recent acute coronary syndrome, dalacetrapib (600 mg daily versus placebo on top of the best available evidence-based care) failed to reduce the risk of recurrent cardiovascular events—death from CAD, nonfatal myocardial infarction, ischemic stroke, unstable angina, or cardiac arrest with resuscitation over a median follow-up of 31 months (121). A post-hoc analysis from this trial showed that subjects with AA genotype of the ADCY9 gene (a gene encoding for adenylyl cyclase) had reduced CVD risk in the dalcetrapib group compared with placebo (122). In the REVEAL trial, anacetrapib showed a modest reduction of cardiovascular events (coronary death, myocardial infarction or coronary revascularization) over a 4.1-year median follow-up. The study included 30,449 adults with atherosclerotic vascular disease who were receiving intensive atorvastatin therapy and had a mean LDL cholesterol of 61 mg/dL. Patients were randomized to anacetrapib (100 mg/day) or placebo. Anacetrapib was associated with a 104% relative increase in the HDL cholesterol level (36% increase in Apo-A1) and 41% decrease in the LDL cholesterol level. The risk for cardiovascular events was reduced by 9% (123). However, it was suggested that the large increase in the HDL level should have been associated with a much greater difference in the risk reduction for coronary events and that the concomitant 41% decrease of LDL cholesterol may have accounted for most if not all reduced CVD risk (11). In the ACCELERATE trial, evacetrapib was investigated on a randomized basis in 12,092 high-risk patients for coronary events. At 3 months, evacetrapib was associated with a 31.1% decrease in LDL cholesterol and 133.2% increase in HDL cholesterol. However, after a median follow-up of 26 months, evacetrapib failed to show any measurable effect on cardiovascular events (cardiovascular death, myocardial infarction, stroke, coronary revascularization, or hospitalization for unstable angina) being 12.9% in the evacetrapib group and 12.8% in the placebo group (P=0.91). The study concluded that evacetrapib did not reduce the risk of cardiovascular events in patients at risk for cardiovascular events (124). A recent meta-analysis including 154,601 patients from 31 randomized trials (with a sample size of ≥100 patients and a follow-up of ≥6 months) showed that the use HDL cholesterol modifiers (fibrates, niacin and CETP inhibitors) had no significant effect on cardiovascular mortality, stroke or all-cause mortality. HDL modifiers reduced the risk for myocardial infarction but meta-regression showed that this benefit was consistent with an absolute reduction in the LDL cholesterol. The statin therapy attenuated the benefit of HDL modifiers on the risk for myocardial infarction (125). In summary, the studies with CETP inhibitors failed to demonstrate a relationship between pharmacologically increased levels of HDL and various cardiovascular outcomes. These studies dealt a blow to the HDL-hypothesis of atherosclerosis and ended the era of using the HDL-rising therapies as a strategy to prevent atherosclerosis and CAD.
Mendelian randomization studies that have assessed cardiovascular events in carriers of genetic variants that are associated with lifelong differences in HDL levels were expected to provide evidence with respect to causality relationship between HDL and CVD. However, so far these studies did not find a lower cardiovascular risk in subjects who are genetically predisposed to high HDL levels. In one Mendelian randomization study, involving polymorphisms of endothelial lipase, carriers of the LIPG 396Ser allele (2.6% frequency) had higher HDL levels (but not other lipids such as LDL or triglycerides) but not reduced CAD risk (126). Another Mendelian analysis identified a homozygote for a loss-of-function variant of the SCARB1 gene, which encodes the SR-BI receptor, in which leucine replaces proline 376 (P376L). Since the posttranslational processing of SR-BI was impaired, selective uptake of HDL was diminished raising the HDL level. The human part of the study showed that subjects who were heterozygous carriers of the P376L variant had significantly increased levels of plasma HDL and were at increased (79% higher) cardiovascular risk (127). However, complexity of genetic pathways makes the interpretation of these studies rather difficult. As shown in a Mendelian randomization analysis of the CETP gene, genetic variants with reduced CETP activity were associated with increased HDL, reduced LDL and decreased risk for atherosclerotic cardiovascular events (128). However, as it was the case for anacetrapib in the REVEAL trial, it remains doubtful whether the reduction in cardiovascular risk is attributable to HDL increase or, more likely, to LDL decrease (123). Nevertheless, the dependence of the cardiovascular effects of CETP inhibitors on genetic profile remains complicated and poorly understood (129). Finally, genome-wide association studies have identified multiple genetic loci that are associated with HDL or Apo-A1 levels but these studies consistently reported no association between genetically-determined HDL and the risk for CAD (126,130-132). Although, genetic studies so far did not support a causal relationship between HDL and atherosclerotic CVD, they have limitations related to summation of a high number of genetic variants with unknown function and to not capturing the functional HDL properties (11).
Other studies investigated the association of HDL particle number, size, cholesterol efflux capacity (CEC) and Apo-A1 as biomarkers of CVD. In the MESA study, HDL cholesterol and HDL particle number correlated inversely with the risk for CAD. However, after bilateral adjustment, HDL particle number remained significantly and inversely associated with the risk for CAD (133). Similarly, in the Dallas Heart Study, HDL particle number was inversely associated with cardiovascular events after adjustment for HDL. Conversely, the association of HDL with cardiovascular events was abolished after adjustment for HDL particle number (134). In the Women’s Health Study, HDL showed a stronger association with CAD than HDL particle number (135). In the JUPITER trial, HDL particle number but not HDL was associated inversely with adverse cardiovascular events (136). In a recent post-hoc analysis from this trial, HDL particle number showed an inverse association with cardiovascular events that was stronger than HDL cholesterol, CEC or Apo-A1 (137). Other population-based studies have reported an inverse association between HDL particle number and the risk for CAD (138-140). Fibrates and statins appear to increase HDL particle number (138,141). In studies that have assessed the association between HDL size, large HDL particles were consistently associated with reduced CVD risk whereas higher levels of small HDL particles were found in patients with confirmed CAD (142,143). However, the assessment of the HDL size depends on the technique used to classify HDL according to size and so far HDL size appears not to help with respect to risk stratification or assessment of HDL-raising strategies. The Dallas Heart Study assessed the association between CEC and atherosclerotic CVD in 2,924 adults free from CVD (at entry) over a median follow-up of 9.4 years. CEC was assessed by measuring the efflux of fluorescence-labeled cholesterol from J774 macrophages to apolipoprotein B-depleted plasma. The study showed no association between HDL and cardiovascular events. After full adjustment, being in the upper CEC quartile was associated with 67% reduction in the risk for incident cardiovascular events compared with being in the lowest CEC quartile (134). Apo-A1 infusions may reduce CVD risk through augmentation of RCT via ABCA1 receptors. In animal (144) and small clinical (145) studies, Apo-A1 infusions were associated with atherosclerotic plaque regression. However, large randomized trials using wild-type or mutated (Milano variant) Apo-A1 failed to demonstrate clinical benefit despite increases in the CEC of up to 90% (146,147). Finally, interventions that improve metabolic or CVD risk profile appear to increase HDL level or improve its functionality. Thus, bariatric surgery appears to improve multiple HDL functions including CEC, capacity to produce NO and inti-inflammatory, anti-oxidant and anti-apoptotic properties (148). In other studies, exercise improved HDL ability to induce NO production in patients with heart failure (149) whereas weight loss with dieting increased HDL particle number (150) and CEC (151). On the other hand, consumption of saturated fat appears to reduce anti-inflammatory properties of HDL and impair endothelial function (152). HDL function may differ according to ethnicity. Thus, in black South African women, HDL anti-oxidant action appears to be higher than in white women and this may protect them against CAD despite having higher rate of obesity and lower circulating HDL (153). Regular alcohol consumption increases HDL level (154). Smoking cessation increases HDL which appears to occur rapidly after cigarette stopping (155).
In summary, although HDL appears to be inversely associated with the risk for CVD, genetic and HDL-raising intervention studies do not support a causal relationship between HDL and CVD dealing a blow to the HDL-hypothesis of atherosclerosis. Furthermore, with the spectacular failure of CETP inhibition to reduce CVD risk despite a large increase in the HDL concentration, the era of HDL-raising therapies as a strategy to reduce CVD risk appears to have ended.
Dysfunctional HDL—mechanisms of generation and physiological consequences
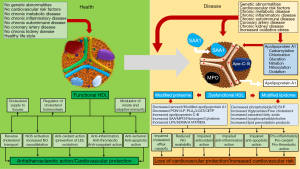
Circulation of HDL in a dysmetabolic environment brought about by morbid conditions such as, heightened inflammatory (and pro-oxidant) states, metabolic diseases (metabolic syndrome or diabetes mellitus), autoimmune diseases, chronic kidney disease, CVD risk factors, coronary atherosclerosis or acute coronary syndrome may lead to structural and functional alterations in the HDL structure transforming HDL from a vasoprotective and anti-atherosclerotic particle into a pro-inflammatory and pro-atherosclerotic dysfunctional equivalent. There are 3 types of changes that may transform HDL from functional to dysfunctional: first, a reduction in the proteome and lipidome constituents that confer to HDL protective vascular and anti-atherosclerotic properties; second increased amount of pro-inflammatory, pro-oxidant and pro-atherosclerotic agents in the HDL particle; and, third, chemical alterations of HDL constituents diminishing their function. Alterations in the HDL composition in various diseases and functional consequences are shown in Figure 3.
In conditions characterized by heightened inflammatory state such as chronic kidney disease, the most evident change in the HDL composition is the accumulation of serum amyloid A (SAA), ApoC-II and ApoC-III associated with reduced content of ApoA-1, ApoA-II, ApoM and PON1 (27). In acute phase responses such as, systemic response to infection, surgery, myocardial infarction or chronic inflammation, HDL is enriched in pro-inflammatory proteins such as, SAA, myeloperoxidase, Apo-C-III and ceruloplasmin, which render the particle pro-inflammatory and pro-atherogenic by limiting its ability to promote CEC (156). In a mixed animal (rabbit) and human study, the impact of acute-phase response on HDL composition and function was investigated. In acute-phase (in rabbits), SAA levels were increased and Apo-A1 levels were decreased by 73% whereas PON1 and platelet activating factor acetylhydrolase activity was reduced by 71% and 90%, respectively. Acute-phase (cardiac surgery) human HDL showed increased ceruloplasmin level and control HDL incubated with ceruloplasmin lost ability to inhibit LDL oxidation. In culture cells (human aortic endothelial and smooth muscle cells), acute-phase HDL induced expression of monocyte chemotactic protein 1 (MCP-1) and acute-phase human HDL obtained 2–3 days after cardiac surgery did not prevent LDL-induced increase in monocyte transmigration, but amplified it by up to 1.8-fold. Conversely, HDL obtained before surgery in the same patients completely inhibited it (157). HDL obtained from patients with CAD showed reduced activity of PON1 enzyme, which normally prevents HDL from oxidation. A HDL-deficient in PON1 may activate protein kinase beta 2 (65), which in turn may cause endothelial dysfunction and insulin resistance in endothelium (158). In particular, the pro-inflammatory and pro-oxidant enzyme myeloperoxidase (released in circulation from activated neutrophils and bound to HDL) produces a variety of reactive species causing APO-A1 oxidation and nitrosylation and impairing its interaction with ABCA1 receptors and consequently CEC and ApoA-1-induced activation of enzymes such as NO-synthase, LCAT and CETP (159,160). These actions profoundly alter HDL functionality. Apo-A1 obtained from human atheroma is heavily oxidized by myeloperoxidase (161) and myeloperoxidase–mediated ApoA-1 oxidation impairs HDL functions including CEC, and anti-oxidant and anti-inflammatory properties (162). HDL obtained from patients with diabetes, stable CAD or acute coronary syndromes or chronic kidney disease fail to activate NO-synthase (or even may inhibit it) and induce NO release in endothelial cells in culture (65,163,164).
Diabetes mellitus has a profound effect on HDL size, composition and function rendering HDL dysfunctional and pro-atherogenic. In patients with diabetes, nonenzymatic glycation of apoA-I occurs via interaction with reactive α-oxoaldehydes impairing cholesterol efflux from macrophages in the arterial wall (165). Apart from Apo-A1, the PON1 enzyme appears to undergo glucation with functional consequences. HDL incubation for one week in 25 mmol/l glucose solution had a significant increase in the glycation products and 65% reduction in PON1 enzymatic activity. In this study, glucated PON1 did not inhibit monocyte adhesion to human aortic endothelial cells in vitro and patients with diabetes and documented CAD had a 40% reduction in PON1 activity (166). In particular, diabetes profoundly modifies HDL lipidome. Thus, patients with type 2 diabetes as compared with nondiabetic controls have increased amounts of triglycerides, saturated fatty acids, free cholesterol, saturated fatty acids, lysophosphatidylcholine and linoleic acid and decreased amounts of cholesteryl esters, phosphplipids (phosphatidylcholine), sphingomyelin, plasmalogen and unsaturated fatty acids (167). Functional HDL had a highly fluid surface, which enables proper functioning of proteins and enzymes, cholesteryl ester migration from surface to the center of the particle (HDL maturation) and cholesteryl ester transfer to other lipoprotein classes (enabling RCT). Increased amounts triglycerides and unsaturated fatty acids and decreased amount of phospholipids and sphingomyelin (compounds consisting of sphingosine, fatty acid and choline- or ethanolamine-phosphate) make the HDL surface rigid and increased HDL surface rigidity contributes to HDL dysfunctionality and impaired RCT (168).
The concept of dysfunctional HDL is important for several reasons. First, transformation of HDL from a protector to a promoter of atherosclerosis may explain the failure of recent epidemiological evidence to support a causal relationship between HDL and atherosclerosis and the downfall of HDL-hypothesis of atherosclerosis. Second, HDL-raising therapies may produce dysfunctional HDL. Thus, CETP pharmacological inhibition by diminishing cholesteryl ester transfer to other lipoproteins may impair RCT, which is one of the most important functions of HDL. In this regard, CETP inhibition may resemble disease-related dysfunctional HDL in which reduced amount or altered function of ApoA-1, LCAT or CETP impairs HDL-induced CEC and RCT. In this regard, the use of CETP inhibitors might have been conceptually wrong. Third, the concept of dysfunctional HDL may be important for future therapies involving HDL. Any future HDL-raising therapy that affects negatively the HDL function(s) may be bound for failure in clinical setting.
From HDL concentration measurement to assessment of functionality – A paradigm shift in HDL studies and future research
The recent advances in the understanding of HDL functions and the failure of HDL-raising therapies to improve clinical outcome despite markedly raising HDL levels has led to a paradigm shift in HDL studies from investigation of HDL quantity (level) to investigation of HDL quality (function). So far, among all HDL functions, the CEC and anti-inflammatory functions, are mostly investigated. Several studies (134,169-172) but not all of them (52) have shown an association between higher CEC and reduced CVD risk. As already mentioned, in the Dallas Heart Study, CEC was inversely associated with the risk for incident cardiovascular events. Of note, CEC in this study did not correlate with obesity or insulin resistance as HDL did, suggesting that factors influencing CEC may differ from those affecting HDL level (134). In a nested case-control sample from the prospective EPIC-Norfolk study, CEC was assessed in 1,745 patients with incident CAD and 1,749 controls free of CVD. CEC correlated positively with HDL concentration, ApoA-1 and alcohol consumption and inversely with type 2 diabetes. After adjustment for a variety of potential confounders, the risk of incident CAD was 20% lower per standard deviation change in CEC (169). In a case-control study, CEC was assessed in 203 healthy volunteers undergoing assessment of carotid artery intima-media thickness, 442 patients with angiography-confirmed CAD and 351 patients without angiography-confirmed CAD. HDL and ApoA-1 correlated significantly with CEC but accounted for less than 40% of its variation. Importantly, CEC correlated inversely with carotid intima–media thickness before and after adjustment for the HDL level. CEC also correlated strongly but inversely with presence of CAD even after adjustment for HDL and Apo-A1 (171). In a recent case-control study from the PREVEND cohort with 351 cases and 354 control subjects, CEC correlated inversely with the adjusted risk for incident cardiovascular events over 12 years of follow-up. CEC correlated positively with HDL and ApoA-1 and negatively with body mass index, C-reactive protein and urinary albumin excretion (172). However, in the Cleveland Clinical Study, higher CEC was associated with higher risk of myocardial infarction and stroke (52). Thus, overall CEC appears to be a promising index to assess the association of HDL function with CVD risk. However, nearly all major studies that have investigated the relationship CEC and CVD risk have used different techniques to quantify it, which makes the standardization of CEC assessment assays in routine clinical practice a priority (12).
Although, available evidence strongly suggests that CEC is inversely associated with adverse cardiovascular events, no study to date has shown an improvement in cardiovascular outcomes by enhancing CEC. Niacin (163) and evacetrapib (173) improve several HDL functions. However, in the light of failure of these drugs to improve clinical outcome in large clinical randomized trials, their use to improve HDL CEC function seems unlikely. Statins appear not to improve HDL CEC (173). Inhibitors of bromodomain and extraterminal (BET) proteins, which act as regulators of gene transcription of Apo-A1, increase ApoA-1 and HDL levels in serum (174). The BET inhibitor apabetalone (RVX00022) improved HDL CEC in monkey (174). In a pooled analysis of 3 small clinical trials with 798 patients with CAD, apabetalone produced dose-dependent changes such as increases in ApoA-1 (by 6.7%), HDL (by 6.5%) and large HDL (by 23.3%) and decrease in C-reactive protein (by 21.1%) compared with placebo. Notably, patients treated with apabetolone showed a reduction in major cardiovascular events (5.9% vs. 10.4% over 220 days of follow-up) which was more prominent in patients with diabetes and those with baseline HDL <39 mg/dL and elevated C-reactive protein (175). However, in the recently published BETonMACE randomized trial that included 2,425 patients with acute coronary syndrome, type 2 diabetes and low HDL-cholesterol level apabetalone (oral 100 mg twice daily) did not significantly reduce the composite endpoint of first cardiovascular death, nonfatal myocardial infarction or stroke (P=0.11) over a median of 26.5 months compared with placebo (176). The infusion of a reconstituted ApoA-1 has been demonstrated to improve CEC compared with placebo in a phase 2 trial (177). The AEGIS-II trial (NCT03473223) involving 17,000 patients with acute coronary syndromes is testing the association of human ApoA-1 (other name: CSL112) infusion with CVD risk on a randomized basis. Finally, the enhancement of LCAT activity through recombinant LCAT, gene therapy or the use of small molecule activators is under preclinical and clinical research as a strategy to promote HDL CEC and reduce CVD risk. Intravenous recombinant LCAT infusion improved CEC in patients with CVD (178). Two recent phase 2 trials showed that single‐ascending‐dose of MEDI6012—a recombinant human LCAT—was safe and well tolerated and induced dose‐dependent increases in the HDL-cholesterol and cholesteryl ester concentration in the HDL particle consistent with the LCAT action in patients with stable coronary heart disease on statin therapy (179,180). Another phase 2 trial using intravenously infused MEDI6012 is ongoing (NCT03578809). The best known small molecule LCAT activators are compound A (3-[5-(ethylthio)-1,3,4-thiadiazol-2-ylthio]pyrazine-2-carbonitrile) derivatives (181,182). Compound A derivatives bind to cysteine residue 31 (Cys-31) in the LCAT active site and raise cholesteryl esters and HDL-cholesterol in plasma in mice and hamsters (181,183). A recent experimental study showed that small molecule LCAT activator DS-8190a enhanced RCT and suppressed atherosclerosis in mice (184). Although the results of preclinical research with these agents are promising, so far there is no evidence on their benefit in clinical setting.
Anti-inflammatory functions are under investigation in the setting of functional HDL assessment, as well. Thus, the HDL inflammatory index (HII) measures the degree of LDL oxidation by cell-free assay and LDL-mediated monocyte chemotactic activity as influenced by HDL compared with control LDL (129). A case-control angiographic study showed that patients with acute coronary syndrome but not those with stable CAD had higher mean values of HII suggesting a potential role of pro-inflammatory HDL in the pathophysiology of acute coronary syndrome (185). In a nested case-control study from the PREVEND study the HDL anti-inflammatory capacity (determined as its ability to suppress tumor necrosis factor α-induced vascular cell adhesion molecule-1 mRNA expression in endothelial cells in vitro) was assessed in 369 cases and 340 controls over a median follow-up of 10.5 years. HDL anti-inflammatory capacity did not correlate with HDL or high-sensitivity C-reactive protein. HDL anti-inflammatory capacity was lower in cases than controls and was inversely associated with incident CVD after full adjustment. Notably, HLD anti-inflammatory capacity did not correlate with CEC and it improved risk prediction by the Framingham risk score (186). In children with chronic kidney disease, HDL-mediated NO bioavailability (another HDL function) was found to correlate with arterial markers such as carotid intima-media thickness and arterial stiffness (assessed by measurement of pulse-wave velocity) (187).
Concluding remarks
Despite decades of intense research, which markedly expanded our understanding on HDL functions, the biological role of this lipoprotein particle remains partially known. Recent research showed that HDL plays a central role in vascular biology, well beyond the RCT. Initial epidemiological evidence supported the existence of an inverse relationship between HDL level and the risk for atherosclerosis and CAD. Although HDL shows an inverse relationship with CVD risk, pharmacologically-based HDL-raising therapies and genetic (Mendelian randomization) studies did not support a causal relationship between HDL and the risk for atherosclerosis and CAD. So far, no HDL-raising therapy that increased HDL level without a concomitant decrease in the LDL level was shown to reduce CVD events. Modification of HDL structure and function in various morbid conditions including inflammatory states, metabolic diseases, chronic kidney disease, CVD risk factors and coronary atherosclerosis transforms HDL from a vasoprotective and anti-athrosclerotic particle into a pro-inflammatory and pro-atherosclerotic dysfunctional equivalent. Recent advances in the understanding of HDL (dys) function in health and disease and failure of HDL-raising therapies to reduce CVD risk despite markedly raising HDL levels have led to a paradigm shift in the HDL studies from investigation of HDL concentration to investigation of its functions. Interventions that improve HDL functions including CEC and anti-inflammatory function appear to be promising, but clinical evidence with them remains limited. Various therapies aiming to improve HDL functions are in clinical use or under development. Future studies are needed to assess the role of HDL in vascular biology and cardiovascular epidemiology, pathophysiology and pharmacology.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jlpm-21-32). GN serves as an unpaid editorial board member of Journal of Laboratory and Precision Medicine from July 2020 to July 2022.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Macheboeuf M. Recherches sur les phosphoaminolipides du sérum sanguin. Nature des phospholipides liés aux albumines du sérum de Cheval à l’état de cenapses acido-précipitables. Bull Soc Chim Biol (Paris) 1929;11:485-503.
- BARR DP. RUSS EM, EDER HA. Protein-lipid relationships in human plasma. II. In atherosclerosis and related conditions. Am J Med 1951;11:480-93. [Crossref] [PubMed]
- GOFMAN JW. LINDGREN FT, ELLIOTT H. Ultracentrifugal studies of lipoproteins of human serum. J Biol Chem 1949;179:973-9. [Crossref] [PubMed]
- GOFMAN JW. Serum lipoproteins and the evaluation of atherosclerosis. Ann N Y Acad Sci 1956;64:590-5. [Crossref] [PubMed]
- Miller GJ, Miller NE. Plasma-high-density-lipoprotein concentration and development of ischaemic heart-disease. Lancet 1975;1:16-9. [Crossref] [PubMed]
- Castelli WP, Doyle JT, Gordon T, et al. HDL cholesterol and other lipids in coronary heart disease. The cooperative lipoprotein phenotyping study. Circulation 1977;55:767-72. [Crossref] [PubMed]
- Miller NE, Thelle DS, Forde OH, et al. The Tromsø heart-study. High-density lipoprotein and coronary heart-disease: a prospective case-control study. Lancet 1977;1:965-8. [Crossref] [PubMed]
- Assmann G, Schulte H, von Eckardstein A, et al. High-density lipoprotein cholesterol as a predictor of coronary heart disease risk. The PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis 1996;124:S11-20. [Crossref] [PubMed]
- Gordon DJ, Probstfield JL, Garrison RJ, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 1989;79:8-15. [Crossref] [PubMed]
- Lüscher TF, Landmesser U, von Eckardstein A, et al. High-density lipoprotein: vascular protective effects, dysfunction, and potential as therapeutic target. Circ Res 2014;114:171-82. [Crossref] [PubMed]
- Sacks FM, Jensen MK. From High-Density Lipoprotein Cholesterol to Measurements of Function: Prospects for the Development of Tests for High-Density Lipoprotein Functionality in Cardiovascular Disease. Arterioscler Thromb Vasc Biol 2018;38:487-99. [Crossref] [PubMed]
- Chiesa ST, Charakida M. High-Density Lipoprotein Function and Dysfunction in Health and Disease. Cardiovasc Drugs Ther 2019;33:207-19. [Crossref] [PubMed]
- Ronsein GE, Vaisar T. Deepening our understanding of HDL proteome. Expert Rev Proteomics 2019;16:749-60. [Crossref] [PubMed]
- Kontush A, Lindahl M, Lhomme M, et al. Structure of HDL: particle subclasses and molecular components. Handb Exp Pharmacol 2015;224:3-51. [Crossref] [PubMed]
- Kontush A, Lindahl M, Lhomme M, et al. Structure of HDL: Particle Subclasses and Molecular Components. In: Eckardstein A, Kardassis D, Editors. High Density Lipoproteins From Biological Understanding to Clinical Exploitation. Heidelberg: Springer Open, 2015:3-51.
- Schwendeman A, Sviridov DO, Yuan W, et al. The effect of phospholipid composition of reconstituted HDL on its cholesterol efflux and anti-inflammatory properties. J Lipid Res 2015;56:1727-37. [Crossref] [PubMed]
- Pourmousa M, Song HD, He Y, et al. Tertiary structure of apolipoprotein A-I in nascent high-density lipoproteins. Proc Natl Acad Sci U S A 2018;115:5163-8. [Crossref] [PubMed]
- Klon AE, Jones MK, Segrest JP, et al. Molecular belt models for the apolipoprotein A-I Paris and Milano mutations. Biophys J 2000;79:1679-85. [Crossref] [PubMed]
- Rysz J, Gluba-Brzózka A, Rysz-Górzyńska M, et al. The Role and Function of HDL in Patients with Chronic Kidney Disease and the Risk of Cardiovascular Disease. Int J Mol Sci 2020;21:601. [Crossref] [PubMed]
- Kontush A, Lhomme M, Chapman MJ. Unraveling the complexities of the HDL lipidome. J Lipid Res 2013;54:2950-63. [Crossref] [PubMed]
- Ben-Aicha S, Badimon L, Vilahur G. Advances in HDL: Much More than Lipid Transporters. Int J Mol Sci 2020;21:732. [Crossref] [PubMed]
- Ren J, Zhang J, Xu N, et al. Signature of circulating microRNAs as potential biomarkers in vulnerable coronary artery disease. PLoS One 2013;8:e80738 [Crossref] [PubMed]
- Wagner J, Riwanto M, Besler C, et al. Characterization of levels and cellular transfer of circulating lipoprotein-bound microRNAs. Arterioscler Thromb Vasc Biol 2013;33:1392-400. [Crossref] [PubMed]
- Vickers KC, Palmisano BT, Shoucri BM, et al. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol 2011;13:423-33. [Crossref] [PubMed]
- Tsui NB, Ng EK, Lo YM. Stability of endogenous and added RNA in blood specimens, serum, and plasma. Clin Chem 2002;48:1647-53. [Crossref] [PubMed]
- Vickers KC, Michell DL. HDL-small RNA Export, Transport, and Functional Delivery in Atherosclerosis. Curr Atheroscler Rep 2021;23:38. [Crossref] [PubMed]
- Marsche G, Heine GH, Stadler JT, et al. Current Understanding of the Relationship of HDL Composition, Structure and Function to Their Cardioprotective Properties in Chronic Kidney Disease. Biomolecules 2020;10:1348. [Crossref] [PubMed]
- Jomard A, Osto E. High Density Lipoproteins: Metabolism, Function, and Therapeutic Potential. Front Cardiovasc Med 2020;7:39. [Crossref] [PubMed]
- Gordon JI, Sims HF, Lentz SR, et al. Proteolytic processing of human preproapolipoprotein A-I. A proposed defect in the conversion of pro A-I to A-I in Tangier's disease. J Biol Chem 1983;258:4037-44. [Crossref] [PubMed]
- Segrest JP, Garber DW, Brouillette CG, et al. The amphipathic alpha helix: a multifunctional structural motif in plasma apolipoproteins. Adv Protein Chem 1994;45:303-69. [Crossref] [PubMed]
- Vuilleumier N, Dayer JM, von Eckardstein A, et al. Pro- or anti-inflammatory role of apolipoprotein A-1 in high-density lipoproteins? Swiss Med Wkly 2013;143:w13781 [Crossref] [PubMed]
- Van Lenten BJ, Wagner AC, Nayak DP, et al. High-density lipoprotein loses its anti-inflammatory properties during acute influenza a infection. Circulation 2001;103:2283-8. [Crossref] [PubMed]
- Han CY, Chiba T, Campbell JS, et al. Reciprocal and coordinate regulation of serum amyloid A versus apolipoprotein A-I and paraoxonase-1 by inflammation in murine hepatocytes. Arterioscler Thromb Vasc Biol 2006;26:1806-13. [Crossref] [PubMed]
- GLOMSET JA. The mechanism of the plasma cholesterol esterification reaction: plasma fatty acid transferase. Biochim Biophys Acta 1962;65:128-35. [Crossref] [PubMed]
- Rousset X, Vaisman B, Amar M, et al. Lecithin: cholesterol acyltransferase--from biochemistry to role in cardiovascular disease. Curr Opin Endocrinol Diabetes Obes 2009;16:163-71. [Crossref] [PubMed]
- Huang R, Silva RA, Jerome WG, et al. Apolipoprotein A-I structural organization in high-density lipoproteins isolated from human plasma. Nat Struct Mol Biol 2011;18:416-22. [Crossref] [PubMed]
- Tall AR. Plasma cholesteryl ester transfer protein. J Lipid Res 1993;34:1255-74. [Crossref] [PubMed]
- Barter PJ, Brewer HB Jr, Chapman MJ, et al. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arterioscler Thromb Vasc Biol 2003;23:160-7. [Crossref] [PubMed]
- Day JR, Albers JJ, Lofton-Day CE, et al. Complete cDNA encoding human phospholipid transfer protein from human endothelial cells. J Biol Chem 1994;269:9388-91. [Crossref] [PubMed]
- Bailey D, Ruel I, Hafiane A, et al. Analysis of lipid transfer activity between model nascent HDL particles and plasma lipoproteins: implications for current concepts of nascent HDL maturation and genesis. J Lipid Res 2010;51:785-97. [Crossref] [PubMed]
- Albers JJ, Vuletic S, Cheung MC. Role of plasma phospholipid transfer protein in lipid and lipoprotein metabolism. Biochim Biophys Acta 2012;1821:345-57. [Crossref] [PubMed]
- Randolph GJ, Miller NE. Lymphatic transport of high-density lipoproteins and chylomicrons. J Clin Invest 2014;124:929-35. [Crossref] [PubMed]
- Zanoni P, Velagapudi S, Yalcinkaya M, et al. Endocytosis of lipoproteins. Atherosclerosis 2018;275:273-95. [Crossref] [PubMed]
- Velagapudi S, Yalcinkaya M, Piemontese A, et al. VEGF-A Regulates Cellular Localization of SR-BI as Well as Transendothelial Transport of HDL but Not LDL. Arterioscler Thromb Vasc Biol 2017;37:794-803. [Crossref] [PubMed]
- Plochberger B, Röhrl C, Preiner J, et al. HDL particles incorporate into lipid bilayers - a combined AFM and single molecule fluorescence microscopy study. Sci Rep 2017;7:15886. [Crossref] [PubMed]
- von Eckardstein A. Implications of torcetrapib failure for the future of HDL therapy: is HDL-cholesterol the right target? Expert Rev Cardiovasc Ther 2010;8:345-58. [Crossref] [PubMed]
- Rosenson RS, Brewer HB Jr, Davidson WS, et al. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation 2012;125:1905-19. [Crossref] [PubMed]
- Graversen JH, Castro G, Kandoussi A, et al. A pivotal role of the human kidney in catabolism of HDL protein components apolipoprotein A-I and A-IV but not of A-II. Lipids 2008;43:467-70. [Crossref] [PubMed]
- Mendivil CO, Furtado J, Morton AM, et al. Novel Pathways of Apolipoprotein A-I Metabolism in High-Density Lipoprotein of Different Sizes in Humans. Arterioscler Thromb Vasc Biol 2016;36:156-65. [Crossref] [PubMed]
- Glomset JA. The plasma lecithins:cholesterol acyltransferase reaction. J Lipid Res 1968;9:155-67. [Crossref] [PubMed]
- Adorni MP, Zimetti F, Billheimer JT, et al. The roles of different pathways in the release of cholesterol from macrophages. J Lipid Res 2007;48:2453-62. [Crossref] [PubMed]
- Li XM, Tang WH, Mosior MK, et al. Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arterioscler Thromb Vasc Biol 2013;33:1696-705. [Crossref] [PubMed]
- Jakobsson T, Treuter E, Gustafsson JÅ, et al. Liver X receptor biology and pharmacology: new pathways, challenges and opportunities. Trends Pharmacol Sci 2012;33:394-404. [Crossref] [PubMed]
- Francis GA, Knopp RH, Oram JF. Defective removal of cellular cholesterol and phospholipids by apolipoprotein A-I in Tangier Disease. J Clin Invest 1995;96:78-87. [Crossref] [PubMed]
- Dietschy JM, Turley SD. Control of cholesterol turnover in the mouse. J Biol Chem 2002;277:3801-4. [Crossref] [PubMed]
- Zhang Y, Zanotti I, Reilly MP, et al. Overexpression of apolipoprotein A-I promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation 2003;108:661-3. [Crossref] [PubMed]
- Rothblat GH, Phillips MC. High-density lipoprotein heterogeneity and function in reverse cholesterol transport. Curr Opin Lipidol 2010;21:229-38. [Crossref] [PubMed]
- Brufau G, Groen AK, Kuipers F. Reverse cholesterol transport revisited: contribution of biliary versus intestinal cholesterol excretion. Arterioscler Thromb Vasc Biol 2011;31:1726-33. [Crossref] [PubMed]
- Azhar S, Leers-Sucheta S, Reaven E. Cholesterol uptake in adrenal and gonadal tissues: the SR-BI and 'selective' pathway connection. Front Biosci 2003;8:s998-1029. [Crossref] [PubMed]
- Yuhanna IS, Zhu Y, Cox BE, et al. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat Med 2001;7:853-7. [Crossref] [PubMed]
- Shaul PW, Mineo C. HDL action on the vascular wall: is the answer NO? J Clin Invest 2004;113:509-13. [Crossref] [PubMed]
- Bardagjy AS, Steinberg FM. Relationship Between HDL Functional Characteristics and Cardiovascular Health and Potential Impact of Dietary Patterns: A Narrative Review. Nutrients 2019;11:1231. [Crossref] [PubMed]
- Drew BG, Fidge NH, Gallon-Beaumier G, et al. High-density lipoprotein and apolipoprotein AI increase endothelial NO synthase activity by protein association and multisite phosphorylation. Proc Natl Acad Sci U S A 2004;101:6999-7004. [Crossref] [PubMed]
- Kuvin JT, Rämet ME, Patel AR, et al. A novel mechanism for the beneficial vascular effects of high-density lipoprotein cholesterol: enhanced vasorelaxation and increased endothelial nitric oxide synthase expression. Am Heart J 2002;144:165-72. [Crossref] [PubMed]
- Besler C, Heinrich K, Rohrer L, et al. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J Clin Invest 2011;121:2693-708. [Crossref] [PubMed]
- Sacre SM, Stannard AK, Owen JS. Apolipoprotein E (apoE) isoforms differentially induce nitric oxide production in endothelial cells. FEBS Lett 2003;540:181-7. [Crossref] [PubMed]
- Seetharam D, Mineo C, Gormley AK, et al. High-density lipoprotein promotes endothelial cell migration and reendothelialization via scavenger receptor-B type I. Circ Res 2006;98:63-72. [Crossref] [PubMed]
- Zhang Q, Yin H, Liu P, et al. Essential role of HDL on endothelial progenitor cell proliferation with PI3K/Akt/cyclin D1 as the signal pathway. Exp Biol Med (Maywood) 2010;235:1082-92. [Crossref] [PubMed]
- Sampietro T, Bigazzi F, Dal Pino B, et al. Increased plasma C-reactive protein in familial hypoalphalipoproteinemia: a proinflammatory condition? Circulation 2002;105:11-4. [Crossref] [PubMed]
- Baker PW, Rye KA, Gamble JR, et al. Ability of reconstituted high density lipoproteins to inhibit cytokine-induced expression of vascular cell adhesion molecule-1 in human umbilical vein endothelial cells. J Lipid Res 1999;40:345-53. [Crossref] [PubMed]
- Murphy AJ, Woollard KJ, Hoang A, et al. High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler Thromb Vasc Biol 2008;28:2071-7. [Crossref] [PubMed]
- Barter PJ, Nicholls S, Rye KA, et al. Antiinflammatory properties of HDL. Circ Res 2004;95:764-72. [Crossref] [PubMed]
- Navab M, Imes SS, Hama SY, et al. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J Clin Invest 1991;88:2039-46. [Crossref] [PubMed]
- Hyka N, Dayer JM, Modoux C, et al. Apolipoprotein A-I inhibits the production of interleukin-1beta and tumor necrosis factor-alpha by blocking contact-mediated activation of monocytes by T lymphocytes. Blood 2001;97:2381-9. [Crossref] [PubMed]
- Robbesyn F, Garcia V, Auge N, et al. HDL counterbalance the proinflammatory effect of oxidized LDL by inhibiting intracellular reactive oxygen species rise, proteasome activation, and subsequent NF-kappaB activation in smooth muscle cells. FASEB J 2003;17:743-5. [Crossref] [PubMed]
- Vohl MC, Neville TA, Kumarathasan R, et al. A novel lecithin-cholesterol acyltransferase antioxidant activity prevents the formation of oxidized lipids during lipoprotein oxidation. Biochemistry 1999;38:5976-81. [Crossref] [PubMed]
- Parthasarathy S, Barnett J, Fong LG. High-density lipoprotein inhibits the oxidative modification of low-density lipoprotein. Biochim Biophys Acta 1990;1044:275-83. [Crossref] [PubMed]
- Mackness MI, Arrol S, Durrington PN. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett 1991;286:152-4. [Crossref] [PubMed]
- Shih DM, Gu L, Xia YR, et al. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature 1998;394:284-7. [Crossref] [PubMed]
- Brites F, Martin M, Guillas I, et al. Antioxidative activity of high-density lipoprotein (HDL): Mechanistic insights into potential clinical benefit. BBA Clin 2017;8:66-77. [Crossref] [PubMed]
- Garner B, Waldeck AR, Witting PK, et al. Oxidation of high density lipoproteins. II. Evidence for direct reduction of lipid hydroperoxides by methionine residues of apolipoproteins AI and AII. J Biol Chem 1998;273:6088-95. [Crossref] [PubMed]
- Kontush A, Chapman MJ. Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol Rev 2006;58:342-74. [Crossref] [PubMed]
- Wu T, Willett WC, Rifai N, et al. Is plasma oxidized low-density lipoprotein, measured with the widely used antibody 4E6, an independent predictor of coronary heart disease among U.S. men and women? J Am Coll Cardiol 2006;48:973-9. [Crossref] [PubMed]
- Tölle M, Pawlak A, Schuchardt M, et al. HDL-associated lysosphingolipids inhibit NAD(P)H oxidase-dependent monocyte chemoattractant protein-1 production. Arterioscler Thromb Vasc Biol 2008;28:1542-8. [Crossref] [PubMed]
- Bonacina F, Pirillo A, Catapano AL, et al. HDL in Immune-Inflammatory Responses: Implications beyond Cardiovascular Diseases. Cells 2021;10:1061. [Crossref] [PubMed]
- Mathison JC, Ulevitch RJ. The clearance, tissue distribution, and cellular localization of intravenously injected lipopolysaccharide in rabbits. J Immunol 1979;123:2133-43. [PubMed]
- Levine DM, Parker TS, Donnelly TM, et al. In vivo protection against endotoxin by plasma high density lipoprotein. Proc Natl Acad Sci U S A 1993;90:12040-4. [Crossref] [PubMed]
- De Nardo D, Labzin LI, Kono H, et al. High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat Immunol 2014;15:152-60. [Crossref] [PubMed]
- Zhu X, Owen JS, Wilson MD, et al. Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J Lipid Res 2010;51:3196-206. [Crossref] [PubMed]
- Wang SH, Yuan SG, Peng DQ, et al. HDL and ApoA-I inhibit antigen presentation-mediated T cell activation by disrupting lipid rafts in antigen presenting cells. Atherosclerosis 2012;225:105-14. [Crossref] [PubMed]
- Yu BL, Wang SH, Peng DQ, et al. HDL and immunomodulation: an emerging role of HDL against atherosclerosis. Immunol Cell Biol 2010;88:285-90. [Crossref] [PubMed]
- Fernandes das Neves M. Batuca JR, Delgado Alves J. The role of high-density lipoprotein in the regulation of the immune response: implications for atherosclerosis and autoimmunity. Immunology 2021;
- Madsen CM, Varbo A, Tybjærg-Hansen A, et al. U-shaped relationship of HDL and risk of infectious disease: two prospective population-based cohort studies. Eur Heart J 2018;39:1181-90. [Crossref] [PubMed]
- Sugano M, Tsuchida K, Makino N. High-density lipoproteins protect endothelial cells from tumor necrosis factor-alpha-induced apoptosis. Biochem Biophys Res Commun 2000;272:872-6. [Crossref] [PubMed]
- Riwanto M, Rohrer L, Roschitzki B, et al. Altered activation of endothelial anti- and proapoptotic pathways by high-density lipoprotein from patients with coronary artery disease: role of high-density lipoprotein-proteome remodeling. Circulation 2013;127:891-904. [Crossref] [PubMed]
- Mineo C, Deguchi H, Griffin JH, et al. Endothelial and antithrombotic actions of HDL. Circ Res 2006;98:1352-64. [Crossref] [PubMed]
- Griffin JH, Kojima K, Banka CL, et al. High-density lipoprotein enhancement of anticoagulant activities of plasma protein S and activated protein C. J Clin Invest 1999;103:219-27. [Crossref] [PubMed]
- Calkin AC, Drew BG, Ono A, et al. Reconstituted high-density lipoprotein attenuates platelet function in individuals with type 2 diabetes mellitus by promoting cholesterol efflux. Circulation 2009;120:2095-104. [Crossref] [PubMed]
- Siebel AL, Heywood SE, Kingwell BA. HDL and glucose metabolism: current evidence and therapeutic potential. Front Pharmacol 2015;6:258. [Crossref] [PubMed]
- Drew BG, Rye KA, Duffy SJ, et al. The emerging role of HDL in glucose metabolism. Nat Rev Endocrinol 2012;8:237-45. [Crossref] [PubMed]
- von Eckardstein A, Widmann C. High-density lipoprotein, beta cells, and diabetes . Cardiovasc Res 2014;103:384-94. [Crossref] [PubMed]
- Manandhar B, Cochran BJ, Rye KA. Role of High-Density Lipoproteins in Cholesterol Homeostasis and Glycemic Control. J Am Heart Assoc 2020;9:e013531 [Crossref] [PubMed]
- Theilmeier G, Schmidt C, Herrmann J, et al. High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation 2006;114:1403-9. [Crossref] [PubMed]
- Tabet F, Vickers KC, Cuesta Torres LF, et al. HDL-transferred microRNA-223 regulates ICAM-1 expression in endothelial cells. Nat Commun 2014;5:3292. [Crossref] [PubMed]
- Ren K, Zhu X, Zheng Z, et al. MicroRNA-24 aggravates atherosclerosis by inhibiting selective lipid uptake from HDL cholesterol via the post-transcriptional repression of scavenger receptor class B type I. Atherosclerosis 2018;270:57-67. [Crossref] [PubMed]
- Gordon T, Castelli WP, Hjortland MC, et al. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med 1977;62:707-14. [Crossref] [PubMed]
- Wilson PW, Abbott RD, Castelli WP. High density lipoprotein cholesterol and mortality. The Framingham Heart Study. Arteriosclerosis 1988;8:737-41. [Crossref] [PubMed]
- Barter P, Gotto AM, LaRosa JC, et al. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med 2007;357:1301-10. [Crossref] [PubMed]
- Emerging Risk Factors Collaboration. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 2009;302:1993-2000. [Crossref] [PubMed]
- Nicholls SJ, Brown H, Bubb K. High-density lipoproteins and cardiovascular disease. EMJ Cardiol 2020. DOI:
10.33590/emjcardiol/20-00038 .10.33590/emjcardiol/20-00038 - Briel M, Ferreira-Gonzalez I, You JJ, et al. Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: systematic review and meta-regression analysis. BMJ 2009;338:b92. [Crossref] [PubMed]
- AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365:2255-67. [Crossref] [PubMed]
- Landray MJ, Haynes R, Armitage J. Niacin for reduction of cardiovascular risk. N Engl J Med 2014;371:1943-4. [PubMed]
- Cannon CP, Shah S, Dansky HM, et al. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N Engl J Med 2010;363:2406-15. [Crossref] [PubMed]
- Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007;357:2109-22. [Crossref] [PubMed]
- Nissen SE, Tardif JC, Nicholls SJ, et al. Effect of torcetrapib on the progression of coronary atherosclerosis. N Engl J Med 2007;356:1304-16. [Crossref] [PubMed]
- Kastelein JJ, van Leuven SI, Burgess L, et al. Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. N Engl J Med 2007;356:1620-30. [Crossref] [PubMed]
- Bots ML, Visseren FL, Evans GW, et al. Torcetrapib and carotid intima-media thickness in mixed dyslipidaemia (RADIANCE 2 study): a randomised, double-blind trial. Lancet 2007;370:153-60. [Crossref] [PubMed]
- Fayad ZA, Mani V, Woodward M, et al. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet 2011;378:1547-59. [Crossref] [PubMed]
- Lüscher TF, Taddei S, Kaski JC, et al. Vascular effects and safety of dalcetrapib in patients with or at risk of coronary heart disease: the dal-VESSEL randomized clinical trial. Eur Heart J 2012;33:857-65. [Crossref] [PubMed]
- Schwartz GG, Olsson AG, Abt M, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med 2012;367:2089-99. [Crossref] [PubMed]
- Tardif JC, Rhainds D, Brodeur M, et al. Genotype-Dependent Effects of Dalcetrapib on Cholesterol Efflux and Inflammation: Concordance With Clinical Outcomes. Circ Cardiovasc Genet 2016;9:340-8. [Crossref] [PubMed]
- HPS3/TIMI55–REVEAL Collaborative Group. Effects of Anacetrapib in Patients with Atherosclerotic Vascular Disease. N Engl J Med 2017;377:1217-27. [Crossref] [PubMed]
- Lincoff AM, Nicholls SJ, Riesmeyer JS, et al. Evacetrapib and Cardiovascular Outcomes in High-Risk Vascular Disease. N Engl J Med 2017;376:1933-42. [Crossref] [PubMed]
- Riaz H, Khan SU, Rahman H, et al. Effects of high-density lipoprotein targeting treatments on cardiovascular outcomes: A systematic review and meta-analysis. Eur J Prev Cardiol 2019;26:533-43. [Crossref] [PubMed]
- Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet 2012;380:572-80. [Crossref] [PubMed]
- Zanoni P, Khetarpal SA, Larach DB, et al. Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science 2016;351:1166-71. [Crossref] [PubMed]
- Johannsen TH, Frikke-Schmidt R, Schou J, et al. Genetic inhibition of CETP, ischemic vascular disease and mortality, and possible adverse effects. J Am Coll Cardiol 2012;60:2041-8. [Crossref] [PubMed]
- Allard-Ratick MP, Kindya BR, Khambhati J, et al. HDL: Fact, fiction, or function? HDL cholesterol and cardiovascular risk. Eur J Prev Cardiol 2021;28:166-173. [Crossref] [PubMed]
- Holmes MV, Asselbergs FW, Palmer TM, et al. Mendelian randomization of blood lipids for coronary heart disease. Eur Heart J 2015;36:539-50. [Crossref] [PubMed]
- White J, Swerdlow DI, Preiss D, et al. Association of Lipid Fractions With Risks for Coronary Artery Disease and Diabetes. JAMA Cardiol 2016;1:692-9. [Crossref] [PubMed]
- Willer CJ, Schmidt EM, Sengupta S, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet 2013;45:1274-83. [Crossref] [PubMed]
- Mackey RH, Greenland P, Goff DC Jr, et al. High-density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: MESA (multi-ethnic study of atherosclerosis). J Am Coll Cardiol 2012;60:508-16. [Crossref] [PubMed]
- Rohatgi A, Khera A, Berry JD, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med 2014;371:2383-93. [Crossref] [PubMed]
- Akinkuolie AO, Paynter NP, Padmanabhan L, et al. High-density lipoprotein particle subclass heterogeneity and incident coronary heart disease. Circ Cardiovasc Qual Outcomes 2014;7:55-63. [Crossref] [PubMed]
- Mora S, Glynn RJ, Ridker PM. High-density lipoprotein cholesterol, size, particle number, and residual vascular risk after potent statin therapy. Circulation 2013;128:1189-97. [Crossref] [PubMed]
- Khera AV, Demler OV, Adelman SJ, et al. Cholesterol Efflux Capacity, High-Density Lipoprotein Particle Number, and Incident Cardiovascular Events: An Analysis From the JUPITER Trial (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin). Circulation 2017;135:2494-504. [Crossref] [PubMed]
- Otvos JD, Collins D, Freedman DS, et al. Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by gemfibrozil therapy in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Circulation 2006;113:1556-63. [Crossref] [PubMed]
- Kuller LH, Grandits G, Cohen JD, et al. Lipoprotein particles, insulin, adiponectin, C-reactive protein and risk of coronary heart disease among men with metabolic syndrome. Atherosclerosis 2007;195:122-8. [Crossref] [PubMed]
- El Harchaoui K, Arsenault BJ, Franssen R, et al. High-density lipoprotein particle size and concentration and coronary risk. Ann Intern Med 2009;150:84-93. [Crossref] [PubMed]
- Rosenson RS, Otvos JD, Hsia J. Effects of rosuvastatin and atorvastatin on LDL and HDL particle concentrations in patients with metabolic syndrome: a randomized, double-blind, controlled study. Diabetes Care 2009;32:1087-91. [Crossref] [PubMed]
- Asztalos BF, Schaefer EJ. High-density lipoprotein subpopulations in pathologic conditions. Am J Cardiol 2003;91:12E-7E. [Crossref] [PubMed]
- Asztalos BF, Schaefer EJ. HDL in atherosclerosis: actor or bystander? Atheroscler Suppl 2003;4:21-9. [Crossref] [PubMed]
- Shah PK, Yano J, Reyes O, et al. High-dose recombinant apolipoprotein A-I(milano) mobilizes tissue cholesterol and rapidly reduces plaque lipid and macrophage content in apolipoprotein e-deficient mice. Potential implications for acute plaque stabilization. Circulation 2001;103:3047-50. [Crossref] [PubMed]
- Nissen SE, Tsunoda T, Tuzcu EM, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA 2003;290:2292-300. [Crossref] [PubMed]
- Nicholls SJ, Puri R, Ballantyne CM, et al. Effect of Infusion of High-Density Lipoprotein Mimetic Containing Recombinant Apolipoprotein A-I Milano on Coronary Disease in Patients With an Acute Coronary Syndrome in the MILANO-PILOT Trial: A Randomized Clinical Trial. JAMA Cardiol 2018;3:806-14. [Crossref] [PubMed]
- Nicholls SJ, Andrews J, Kastelein JJP, et al. Effect of Serial Infusions of CER-001, a Pre-β High-Density Lipoprotein Mimetic, on Coronary Atherosclerosis in Patients Following Acute Coronary Syndromes in the CER-001 Atherosclerosis Regression Acute Coronary Syndrome Trial: A Randomized Clinical Trial. JAMA Cardiol 2018;3:815-22. [Crossref] [PubMed]
- Osto E, Doytcheva P, Corteville C, et al. Rapid and body weight-independent improvement of endothelial and high-density lipoprotein function after Roux-en-Y gastric bypass: role of glucagon-like peptide-1. Circulation 2015;131:871-81. [Crossref] [PubMed]
- Adams V, Besler C, Fischer T, et al. Exercise training in patients with chronic heart failure promotes restoration of high-density lipoprotein functional properties. Circ Res 2013;113:1345-55. [Crossref] [PubMed]
- Williams PT, Krauss RM, Vranizan KM, et al. Changes in lipoprotein subfractions during diet-induced and exercise-induced weight loss in moderately overweight men. Circulation 1990;81:1293-304. [Crossref] [PubMed]
- Hernáez Á, Castañer O, Elosua R, et al. Mediterranean Diet Improves High-Density Lipoprotein Function in High-Cardiovascular-Risk Individuals: A Randomized Controlled Trial. Circulation 2017;135:633-43. [Crossref] [PubMed]
- Nicholls SJ, Lundman P, Harmer JA, et al. Consumption of saturated fat impairs the anti-inflammatory properties of high-density lipoproteins and endothelial function. J Am Coll Cardiol 2006;48:715-20. [Crossref] [PubMed]
- Woudberg NJ, Goedecke JH, Blackhurst D, et al. Association between ethnicity and obesity with high-density lipoprotein (HDL) function and subclass distribution. Lipids Health Dis 2016;15:92. [Crossref] [PubMed]
- Linn S, Carroll M, Johnson C, et al. High-density lipoprotein cholesterol and alcohol consumption in US white and black adults: data from NHANES II. Am J Public Health 1993;83:811-6. [Crossref] [PubMed]
- Forey BA, Fry JS, Lee PN, et al. The effect of quitting smoking on HDL-cholesterol - a review based on within-subject changes. Biomark Res 2013;1:26. [Crossref] [PubMed]
- Kosmas CE, Martinez I, Sourlas A, et al. High-density lipoprotein (HDL) functionality and its relevance to atherosclerotic cardiovascular disease. Drugs Context 2018;7:212525 [Crossref] [PubMed]
- Van Lenten BJ, Hama SY, de Beer FC, et al. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J Clin Invest 1995;96:2758-67. [Crossref] [PubMed]
- Naruse K, Rask-Madsen C, Takahara N, et al. Activation of vascular protein kinase C-beta inhibits Akt-dependent endothelial nitric oxide synthase function in obesity-associated insulin resistance. Diabetes 2006;55:691-8. [Crossref] [PubMed]
- Ndrepepa G. Myeloperoxidase - A bridge linking inflammation and oxidative stress with cardiovascular disease. Clin Chim Acta 2019;493:36-51. [Crossref] [PubMed]
- Smith JD. Myeloperoxidase, inflammation, and dysfunctional high-density lipoprotein. J Clin Lipidol 2010;4:382-8. [Crossref] [PubMed]
- Huang Y, DiDonato JA, Levison BS, et al. An abundant dysfunctional apolipoprotein A1 in human atheroma. Nat Med 2014;20:193-203. [Crossref] [PubMed]
- Fisher EA, Feig JE, Hewing B, et al. High-density lipoprotein function, dysfunction, and reverse cholesterol transport. Arterioscler Thromb Vasc Biol 2012;32:2813-20. [Crossref] [PubMed]
- Sorrentino SA, Besler C, Rohrer L, et al. Endothelial-vasoprotective effects of high-density lipoprotein are impaired in patients with type 2 diabetes mellitus but are improved after extended-release niacin therapy. Circulation 2010;121:110-22. [Crossref] [PubMed]
- Speer T, Rohrer L, Blyszczuk P, et al. Abnormal high-density lipoprotein induces endothelial dysfunction via activation of Toll-like receptor-2. Immunity 2013;38:754-68. [Crossref] [PubMed]
- Cochran BJ, Ong KL, Manandhar B, et al. High Density Lipoproteins and Diabetes. Cells 2021;10:850. [Crossref] [PubMed]
- Hedrick CC, Thorpe SR, Fu MX, et al. Glycation impairs high-density lipoprotein function. Diabetologia 2000;43:312-20. [Crossref] [PubMed]
- Kostara CE, Ferrannini E, Bairaktari ET, et al. Early Signs of Atherogenic Features in the HDL Lipidomes of Normolipidemic Patients Newly Diagnosed with Type 2 Diabetes. Int J Mol Sci 2020;21:8835. [Crossref] [PubMed]
- Rosenson RS, Brewer HB Jr, Ansell B, et al. Translation of high-density lipoprotein function into clinical practice: current prospects and future challenges. Circulation 2013;128:1256-67. [Crossref] [PubMed]
- Saleheen D, Scott R, Javad S, et al. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol 2015;3:507-13. [Crossref] [PubMed]
- Ritsch A, Scharnagl H, März W. HDL cholesterol efflux capacity and cardiovascular events. N Engl J Med 2015;372:1870-1. [PubMed]
- Khera AV, Cuchel M, de la Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 2011;364:127-35. [Crossref] [PubMed]
- Ebtehaj S, Gruppen EG, Bakker SJL, et al. HDL (High-Density Lipoprotein) Cholesterol Efflux Capacity Is Associated With Incident Cardiovascular Disease in the General Population. Arterioscler Thromb Vasc Biol 2019;39:1874-83. [Crossref] [PubMed]
- Nicholls SJ, Ruotolo G, Brewer HB, et al. Cholesterol Efflux Capacity and Pre-Beta-1 HDL Concentrations Are Increased in Dyslipidemic Patients Treated With Evacetrapib. J Am Coll Cardiol 2015;66:2201-10. [Crossref] [PubMed]
- Bailey D, Jahagirdar R, Gordon A, et al. RVX-208: a small molecule that increases apolipoprotein A-I and high-density lipoprotein cholesterol in vitro and in vivo. J Am Coll Cardiol 2010;55:2580-9. [Crossref] [PubMed]
- Nicholls SJ, Ray KK, Johansson JO, et al. Selective BET Protein Inhibition with Apabetalone and Cardiovascular Events: A Pooled Analysis of Trials in Patients with Coronary Artery Disease. Am J Cardiovasc Drugs 2018;18:109-15. [Crossref] [PubMed]
- Ray KK, Nicholls SJ, Buhr KA, et al. Effect of Apabetalone Added to Standard Therapy on Major Adverse Cardiovascular Events in Patients With Recent Acute Coronary Syndrome and Type 2 Diabetes: A Randomized Clinical Trial. JAMA 2020;323:1565-73. [Crossref] [PubMed]
- Michael Gibson C, Korjian S, Tricoci P, et al. Safety and Tolerability of CSL112, a Reconstituted, Infusible, Plasma-Derived Apolipoprotein A-I, After Acute Myocardial Infarction: The AEGIS-I Trial (ApoA-I Event Reducing in Ischemic Syndromes I). Circulation 2016;134:1918-30. [Crossref] [PubMed]
- Shamburek RD, Bakker-Arkema R, Shamburek AM, et al. Safety and Tolerability of ACP-501, a Recombinant Human Lecithin:Cholesterol Acyltransferase, in a Phase 1 Single-Dose Escalation Study. Circ Res 2016;118:73-82. [Crossref] [PubMed]
- Bonaca MP, George RT, Morrow DA, et al. Recombinant human Lecithin-Cholesterol acyltransferase in patients with atherosclerosis: Phase 2a primary results and phase 2b design. Eur Heart J Cardiovasc Pharmacother 2021; Epub ahead of print. [Crossref] [PubMed]
- George RT, Abuhatzira L, Stoughton SM, et al. MEDI6012: Recombinant Human Lecithin Cholesterol Acyltransferase, High-Density Lipoprotein, and Low-Density Lipoprotein Receptor-Mediated Reverse Cholesterol Transport. J Am Heart Assoc 2021;10:e014572 [Crossref] [PubMed]
- Freeman LA, Demosky SJ Jr, Konaklieva M, et al. Lecithin:Cholesterol Acyltransferase Activation by Sulfhydryl-Reactive Small Molecules: Role of Cysteine-31. J Pharmacol Exp Ther 2017;362:306-18. [Crossref] [PubMed]
- Niemelä A, Koivuniemi A. Positive allosteric modulators of lecithin: Cholesterol acyltransferase adjust the orientation of the membrane-binding domain and alter its spatial free energy profile. PLoS Comput Biol 2021;17:e1008426 [Crossref] [PubMed]
- Chen Z, Wang SP, Krsmanovic ML, et al. Small molecule activation of lecithin cholesterol acyltransferase modulates lipoprotein metabolism in mice and hamsters. Metabolism 2012;61:470-81. [Crossref] [PubMed]
- Sasaki M, Delawary M, Sakurai H, et al. Novel LCAT (Lecithin:Cholesterol Acyltransferase) Activator DS-8190a Prevents the Progression of Plaque Accumulation in Atherosclerosis Models. Arterioscler Thromb Vasc Biol 2021;41:360-76. [PubMed]
- Patel PJ, Khera AV, Jafri K, et al. The anti-oxidative capacity of high-density lipoprotein is reduced in acute coronary syndrome but not in stable coronary artery disease. J Am Coll Cardiol 2011;58:2068-75. [Crossref] [PubMed]
- Jia C, Anderson JLC, Gruppen EG, et al. High-Density Lipoprotein Anti-Inflammatory Capacity and Incident Cardiovascular Events. Circulation 2021;143:1935-45. [Crossref] [PubMed]
- Shroff R, Speer T, Colin S, et al. HDL in children with CKD promotes endothelial dysfunction and an abnormal vascular phenotype. J Am Soc Nephrol 2014;25:2658-68. [Crossref] [PubMed]
Cite this article as: Ndrepepa G. High-density lipoprotein: a double-edged sword in cardiovascular physiology and pathophysiology. J Lab Precis Med 2021;6:28.