
Specific leukocyte differential and morphological alarms a clue for the detection of SARS-CoV-2 infection
Complete blood count (CBC) and leukocyte differential in COVID-19 patients present unique characteristics; in one hand neutrophilia, which is most commonly associated to an acute bacterial infection, and in the other lymphopenia, in contrast with lymphocytosis occurring most of the acute viral infections (1). The examination of peripheral blood film reveals morphological anomalies with pleomorphic atypical lymphocytes. Those reactive lymphocytes are usually seen in other viral infections, and plasmacytoid lymphocytes are also frequently present (2-7). The proportion of activated lymphocytes is associated to severe cases (8).
Modern blood cell counters have become increasingly sophisticated; in the new generation manufacturers apply innovative analytical principles to produce both cell counts and morphological analysis. The presence of abnormal cells, such as reactive lymphocytes, are indicated by the generation of alarm flags.
The technologies applied to the counters include impedance, radiofrequency (conductivity) which measures intracellular properties like nucleus/cytoplasm ratio, nuclear density and granularity; chemical treatment uses dedicated fluorescent dyes for staining nuclei and cellular contents; laser light scatter depends on cellular properties like granularity, nuclear density, surface, etc. (9).
The leukocyte differentials can be evaluated in cytograms, where the presence of activated reactive lymphocytes can be detected, flagged and, in certain brands, quantified (10). But this qualitative information presents high heterogeneity because they are absolutely dependent on technique and instrument. The prominent quantitative and qualitative changes of leukocytes in response to SARS-CoV-2 infection can be recognized in the scatterplots of diverse brand counters (Figure 1).
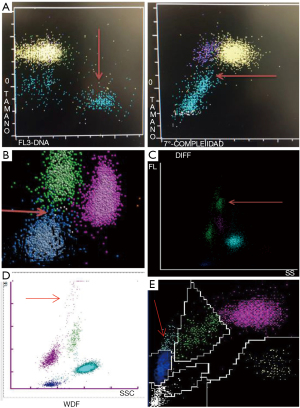
A brief review of the technology in use by manufacturers
Abbott (Chicago, Ill, USA)
The leukocyte count and differential on the CELL-DYN SapphireTM is done using Multi Angle Polarized Scatter Separation (MAPSSTM) and 3-color fluorescent technology. Multiple scatterplots are produced for further identification and enumeration of leukocytes (WBC) subpopulations and abnormal cell types; up to 20,000 cells identified and classified.
In the WBC differential plot the 0° scatter (representing cell size) is plotted against the 7° scatter (cell complexity). Region 2 is of high clinical importance, as pathological cells such as atypical lymphocytes may reside in this region.
Reactive lymphocytes may display in a separate area in the Low-Hi FL plot, a graph of cell size (0° scatter) against fluorescence (FL3).
Beckman Coulter (Brea, CA, USA)
The technology applied is VCS (volume, conductivity and scatter). Nearly 8,000 cells in their “near native state” are analyzed and classified according to volume (V, using direct current impedance), the cellular internal composition (C, conductivity, measured by radiofrequency) and the cytoplasmic granularity and structure of the nucleus (S, light scatter is measured by a laser beam).
The mean and standard deviation of volume, conductivity, and 5‐angle light scattering parameters are reported, corresponding to 14 parameters related to the morphology of leukocytes.
The BC-6800 Plus system (Mindray, Shenzhen, China)
Cells are counted and classified in diverse analytical channels, using different reagents; channel-specific light detectors in certain angles for each cell, give information on cell size, cytoplasmic structures, and nuclear characteristics (S-Cube technology).
The surfactant causes the hemolysis of erythrocytes and platelets perforating the cell membranes of leukocytes. Next, the fluorescence reagent penetrates the leukocytes and labels the nucleic acids and the cytoplasmic organelles. After incubation, the sample is analyzed using the semiconductor laser and measuring the forward and side scatter fluorescent optical signals.
Siemens (Erlangen, Germany)
The peroxidase and the lobularity/nuclear density methods are used. Red cells are lysed and peroxidase reagents can distinguish between peroxidase-positive cells (neutrophils, eosinophils, and monocytes) and peroxidase-negative cells, including lymphocytes, basophils, and “large unstained cells” (LUCs). In this area the reactive lymphocytes can be detected.
A tungsten-based optical system is used to count all leukocytes and to determine the absorbance (stain intensity) and cell size (by forward light scatter) for each cell. The cells absorb light in proportion to the amount of peroxidase stain present, and this peroxidase activity parameter is plotted on the x-axis of the peroxidase cytogram. Cells scatter light in proportion to their size, and is represented along the y-axis of the cytogram.
Sysmex Corporation (Kobe Japan)
The principles are similar to those described for Mindray counters. Using flow cytometry forward scatter (FSC), side scatter (SSC) and side fluorescence (SFL) optical signals are recorded. The intensities of the optical signals FSC and SSC reflect cell surface structure, shape and size, and internal granules of the leukocytes. SFL reflects the number of nucleic acids and cell organelles. When present, the reactive lymphocytes appear in a characteristic cluster in the y-axis in the scatterplot can be quantified.
During the time of the COVID-19 outbreak the Emergency Department has become hospitals’ first line of defense and the most important department for receiving and screening suspected cases. COVID-19 could begin with flu-like symptoms. Currently, clinicians suspect to be dealing with the disease when a patient presents cough, shortness of breath or fever. The current gold standard for the etiological assessment is real‐time reverse transcription‐polymerase chain reaction from oral or nasopharyngeal swab specimens, but this is time consuming and a fast initial indication of COVID-19 would be beneficial to start not only the treatment faster, also the isolation and safety procedures to reduce the risk of transmission of the infection.
It is mandatory to implement better systems for “watchful waiting” of suspected cases and CBC could aid for this purpose: it is the laboratory test most frequently ordered by emergency physicians; analyzed upon admission, the results are often available within the hour; cheap and fast, the information is available 24/7 in hospitals of all level.
Leukocyte differential, the morphological alarms and the evaluation of the scatterplots could assist in a preliminary diagnosis of the disease. The flip side involves the variability of the counters, a fact that makes it essential to learn the technology and capabilities of the analyzers of different manufacturers, for the effective evaluation of the information in the scattergrams.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was a standard submission to the journal. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jlpm-21-26). Dr. EU serves as an unpaid editorial board member of Journal of Laboratory and Precision Medicine from Oct 2019 to Sep 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Karimi Shahri M, Niazkar HR, Rad F. COVID-19 and hematology findings based on the current evidences: A puzzle with many missing pieces. Int J Lab Hematol 2021;43:160-8. [Crossref] [PubMed]
- Zini G, Bellesi S, Ramundo F, et al. Morphological anomalies of circulating blood cells in COVID-19. Am J Hematol 2020;95:870-2. [Crossref] [PubMed]
- Foldes D, Hinton R, Arami S, et al. Plasmacytoid lymphocytes in SARS-CoV-2 infection (Covid-19). Am J Hematol 2020;95:861-2. [Crossref] [PubMed]
- Gérard D, Henry S, Thomas B. SARS-CoV-2: a new aetiology for atypical lymphocytes. Br J Haematol 2020;189:845. [Crossref] [PubMed]
- Chong VCL, Lim KGE, Fan BE, et al. Reactive lymphocytes in patients with COVID-19. Br J Haematol 2020;189:844. [Crossref] [PubMed]
- Weinberg SE, Behdad A, Ji P. Atypical lymphocytes in peripheral blood of patients with COVID-19. Br J Haematol 2020;190:36-9. [Crossref] [PubMed]
- Wang Z, He Y, Shu H, et al. High-fluorescent lymphocytes are increased in patients with COVID-19. Br J Haematol 2020;190:e76-8. [Crossref] [PubMed]
- Pozdnyakova O, Connell NT, Battinelli EM, et al. Clinical Significance of CBC and WBC Morphology in the Diagnosis and Clinical Course of COVID-19 Infection. Am J Clin Pathol 2021;155:364-75. [Crossref] [PubMed]
- Hoffman J, Llewellyn-Smith N. Challenges in hematology diagnostics. MLO Med Lab Obs 2014;46:8-9. [PubMed]
- Osman J, Lambert J, Templé M, et al. Rapid screening of COVID-19 patients using white blood cell scattergrams, a study on 381 patients. Br J Haematol 2020;190:718-22. [Crossref] [PubMed]
Cite this article as: Urrechaga E, Bóveda O, Hernández M, García San Vicente B, Fernández M, Redín H, Pérez I, Morales-Indiano C, Alcaide MJ, García de Guadiana L. Specific leukocyte differential and morphological alarms a clue for the detection of SARS-CoV-2 infection. J Lab Precis Med 2021;6:32.


