
Iron deficiency in chronic inflammatory bowel diseases: an update
Introduction
Iron deficiency (ID) is the most widespread nutritional deficiency in the world. The ID appears first without anemia, and later, microcytic hypochromic anemia can occur. Iron deficiency could affect more than 1.2 billion affected individuals with anemia and probably more than double without anemia (1).
Inflammatory bowel diseases (IBD) are common gastrointestinal diseases that include Crohn’s disease (CD), ulcerative colitis (UC), and inflammatory bowel disease unclassified (IBDU). Those chronic diseases share as a common trait inflammation of the wall of part of the gastrointestinal tract, associated with abdominal pain, frequent and possibly bloody diarrhea, sometimes anal lesions, and other extra-digestive manifestations (notably articular, cutaneous, ocular, hepatic). The diagnosis of IBD is currently multidisciplinary, based on clinical, biological, and medical imaging criteria. The incidence of IBD has been shown to increase worldwide over time. IBD thus represent significant health and economic burden for developed countries (2). More than 2.5 million European individuals and 1.5 million in the USA suffer from these diseases (3). These diseases are frequently identified in young people ranging from 20 to 30 years (4). However, those diseases can occur at any age, notably earlier, since 15% of patients are children (5).
IBD are characterized by inflammatory episodes, varying in intensity and duration, and alternating with remissions. The inflammatory disease can affect the whole digestive tract in CD, from the mouth to the rectum. Most frequently, CD affects only the small intestine and the beginning of the large intestine. These lesions occur by patches and leave healthy mucosa pieces between inflammatory areas. Conversely, in UC, injuries are continuous and generally range between the colon to the rectum. The origins of IBD are complex, and the etiology of IBD is only partly understood. The family aggregation has long been identified (2), with a polygenic heritability suggested. A genetic predisposition and a dysregulation of the immune system have been identified in the pathophysiological process. Moreover, some environmental factors, including the microbiome, could also participate in the genesis of the disease.
Iron deficiency
Iron is the essential component of hemoglobin, mainly found in the erythrocytes, and myoglobin in muscles, which contain around 80% of total body iron. The iron is also mandatory for cellular respiration, energy production, DNA synthesis, and cell proliferation (6,7), mainly as a prosthetic group in hemoproteins. Iron deficiency is due to an insufficient supply of iron to meet the requirements of the body. The daily loss is estimated to be within 1–2 mg/day. In its most evolved form, iron deficiency is associated with microcytic anemia (8). Iron deficiency with or without anemia can is related to other non-hematologic symptoms. Iron deficiency may be related to fatigue, a negative impact on life’s quality, a productivity decrease (9,10), and delayed growth and development in children (11). Iron deficiency is a significant public health problem because undiagnosed and untreated and causes more than 60% of anemia worldwide. All-cause anemia is associated with increased morbidity and mortality in patients affected by inflammatory gastrointestinal diseases, cancer or kidney failure, and during and after surgery (12,13).
Absolute and functional ID are the two main clinic-biological profiles associated with the defect. Both are currently observed in IBD. A decrease in the total iron supply in the organism characterized absolute iron deficiency. It is related either to an insufficient iron intake or chronic blood loss, or, eventually, both. Functional iron deficiency is due to a defect in iron transport from the storage areas. A persistent inflammatory state with increased cytokines, particularly interleukin 6, together with the inappropriate increase of hepcidin, is the primary etiology of this functional deficiency (14). Hepcidin is a polypeptide synthesized primarily in the liver. This peptide is an acute-phase reactant that regulates the plasma iron concentration at the systemic level.
Hepcidin induces degradation by the internalization of ferroportin transporter. Ferroportin is also known as solute carrier family 40 member 1 (SLC40A1) or iron-regulated transporter 1 (IREG1). This transporter is a transmembrane protein. It is the only known iron exporter that permits iron transfer from intracellular to extracellular medium (15).
Absolute ID in patients with IBD could be due to increased loss and decreased intakes of iron: continuous or recurrent blood loss from the bowel and reduced iron intake, and limited iron absorption from the digestive system. Moreover, chronic inflammation should be associated with increased hepcidin, leading to a functional iron deficiency associated with absolute iron deficiency.
Iron deficiency in IBD
ID is probably underdiagnosed and undertreated in IBD in current clinical practice (16). The most frequent etiology of iron deficiency in patients with IBD should be an increased iron loss due to gastrointestinal blood loss. However, this absolute iron deficiency can also be related to decreased absorption and the pro-inflammatory climate associate with an increased interleukin six concentration.
In a multicentric Scandinavian study, 35% of IBD patients were iron deficient (17), 45% in patients with UC (18). This percentage rises as high as 76% in other cohorts (19), making iron deficiency the most common systemic complication of IBD.
However, iron deficiency is generally not systematically screened for in IBD patients. Fatigue, although not specific to iron deficiency, is a prevalent and disabling symptom in patients with IBD, and it is well-known that it is associated with iron deficiency. Nearly 80% of those patients with active disease and 50% with inactive IBD report substantial fatigue.
In a recent Spanish study, the prevalence of ID in IBD patients was as high as 37% (OR =2.9; P=0.015) (20). Iron deficiency was mostly observed in females. The presence of inflammatory activity (OR =9.4; P=0.001) was the main determinant factor associated with an iron deficiency, suggesting that the functional deficiency was more important than absolute iron deficiency, as previously evoked (21).
Anemia in IBD
Anemia is prevalent in patients with IBD: 14–19% of all patients with IBD are anemic, and 20–54% were shown to be deficient in iron (22). In UC, the prevalence was estimated at 66% in inpatients (23) and 40% in reference centers (24).
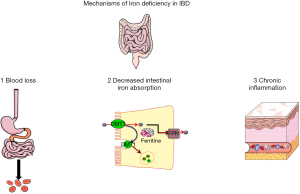
Several factors contribute to iron deficiency and anemia, including intestinal blood loss (macroscopic or microscopic), insufficient iron intake, reduced iron absorption, altered iron metabolism and storage, and the inhibitory effect of pro-inflammatory cytokines on erythropoiesis and iron-binding (25,26), as presented in Figure 1. Anemia related to chronic inflammation and iron deficiency anemia are the two most common causes of anemia in patients with IBD.
In a prospective study of patients with IBD, Jelsness-Jørgensen et al. showed that anemia was associated with chronic fatigue for at least six months (27).
Diagnosis of iron deficiency in IBD
The consensus published in 2015 on anemia management in IBD mentioned the following assays: complete blood count (because of diagnosis and classification of anemia), serum ferritin, and C-reactive protein to detect inflammation (28). The determination of iron deficiency anemia in patients with IBD is complicated: chronic inflammation with or without functional iron deficiency can overlap. Chronic inflammation can impact the values of iron metabolism proteins, as these are also acute-phase proteins. Serum ferritin is the most common biological exam to evaluate iron storage. Its primary disadvantage is that it is an acute-phase protein that can be increased in the setting of inflammation.
Absolute ID is characterized by a low blood ferritin concentration and a low transferrin saturation (TSAT) index. Opposingly, functional iron deficiency is characterized by a normal-to-high serum ferritin concentration and a low TSAT (29). Iron is mainly stored in the body as ferritin in the liver. The serum iron, bound to transferrin, is present in the blood circulation, resulting in release from macrophages and RBC lysis. TSAT (%) is the ratio of serum iron concentration (µmol/L)/total iron-binding capacity, TIBC; (µmol/L), after deduction of the serum concentration of transferrin (g/L). TSAT is considered the best indicator of iron reserves in the bone marrow, and a percentage below 16% confirms iron-deficient anemia (29). It should be mentioned that circadian changes in iron concentration cause fluctuations in TSAT and transferrin synthesis (30).
During inflammation, the lower limit of serum ferritin consistent with regular iron stores is generally assumed to be 100 µg/L. In this setting, the diagnostic criterium is serum ferritin less than 100 µg/L and transferrin saturation less than 20 percent. If the serum ferritin concentration is between 30 and 100 µg/L, a combination of true iron deficiency and functional iron deficiency is likely (18).
Thus, in IBD, ferritin measurement must be associated with the TSAT since inflammation led to a functional iron deficiency. Indeed, increases in hepcidin concentrations are induced by inflammatory cytokines, especially interleukin 6 (31,32).
In an unclear pattern, the soluble transferrin receptor (sTfR), a soluble monomer whose concentration could be measured in the blood (33). This truncated form circulates and forms complexes with transferrin. sTfR concentration is proportional to that of the transferrin receptor on erythropoietic cells. The level of sTfR is decreased during decreased erythropoietic activity. On the contrary, soluble TfR concentrations increased when erythropoiesis is enhanced, for instance, by hemolysis or ineffective erythropoiesis (34).
sTfR has the advantage of being insensitive to inflammation in contrast to ferritin (positive inflammation protein) and transferrin (negative inflammation protein) and entirely independent from hepatic function. Blood sTfR increases in proportion to iron requirements, depletion of iron stores, and increased transferrin synthesis by erythropoietic cells. To better evaluate body iron supplies, it has been proposed to report ferritin’s value, particularly in logarithmic form: sTfR/log (ferritin). This ratio should be very high in iron deficiency by increased sTfR and decreased ferritin. This ratio has been proposed to be more sensitive than the sTfR alone and to reflect both the iron pool and the iron storage (35-37).
Zinc protoporphyrin (ZnPP) in circulating erythrocytes has historically been used as a marker of iron deficiency. In the last step of the heme biosynthesis, protoporphyrin IX is combined with Fe2+ to constitute the heme molecule (38). In the case of iron deficiency, Zn2+ replaces Fe2+ to produce ZnPP. The standard ratio of iron to zinc in protoporphyrin is approximately around 30,000:1, but ZnPP will increase to measurable concentrations with progressive iron deficiency. ZnPP production is unaffected by chronic inflammation and is a helpful indicator of iron deficiency in chronic inflammatory disease. ZnPP/heme ration <40 µmol/mol is a common value in the general population. ZnPP range between 40–80 µmol/mol heme represents latent iron deficiency (normal hemoglobin level), and values >80 µmol/mol heme are associated with manifest iron deficiency (39).
General consideration for the treatment of iron deficiency in IBD
Dietary iron is available either in hemic and non-hemic-forms. Heme iron form as hemoglobin contains ferrous ion Fe2+ in complex with protoporphyrin ring. Its absorption by enterocytes is higher, although this form is less represented in the food than the non-hemic form. Most iron in the diet is inorganic (Fe3+ or ferric ion) from plant-source foods. Organic iron constitutes 10–15% of total iron ingestion, but it represents more than 30% of the total absorbed iron (33) because of its higher bioavailability.
Anti-inflammatory therapy could help resolve the iron sequestration and could be helpful in the management of functional iron deficiency; anemia may, however, be back fast after successful treatment, due notably to absolute ID. Iron absorption has been shown to correlate with inflammation in a recent study (40).
Oral iron supplementation may potentiate gastrointestinal side effects; moreover, the malabsorption of oral iron is also suspected. Consequently, intravenous administration of iron is the first-line treatment in patients with clinically active IBD, intolerance to oral iron, or hemoglobin concentration less than 10 g/dL. IV iron is active, shows a faster response, and is better tolerated than oral iron in these patients (28).
Conclusion
The prevalence of ID, either absolute or functional, appears relatively high in IBD patients. Due to the inflammatory climate, this disease has to be accurately diagnosed, using TSAT rather than ferritin, and to be treated appropriately. Peyrin-Biroulet et al. proposed a three-step strategy (early detection and intervention, treating-to-target, and tight monitoring) to the management of iron deficiency in IBD patients (10). This management could be a way to easily and quickly improve the quality of life and, notably, fatigue in IBD patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by editorial office, Journal of Laboratory and Precision Medicine for the series “Physiology and Pathology of Iron Metabolism”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm-21-49). The series “Physiology and Pathology of Iron Metabolism” was commissioned by the editorial office without any funding or sponsorship. KP served as the Guest Editor of the series and serves as an unpaid editorial board member of Journal of Laboratory and Precision Medicine from July 2019 to June 2021. KP reports some fees for consulting and educational events for Vifor France. The authors have no other conflicts of interest to declare.
Ethical Statement: All authors are accountable for all aspects of this work in ensuring that questions related to the accuracy or integrity of any part of this work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mota JO, Tounian P, Guillou S, et al. Estimation of the Burden of Iron Deficiency Anemia in France from Iron Intake: Methodological Approach. Nutrients 2019;11:2045. [Crossref] [PubMed]
- Momozawa Y, Dmitrieva J, Théâtre E, et al. IBD risk loci are enriched in multigenic regulatory modules encompassing putative causative genes. Nat Commun 2018;9:2427. [Crossref] [PubMed]
- Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol 2015;12:720-7. [Crossref] [PubMed]
- Winter DA, Karolewska-Bochenek K, Lazowska-Przeorek I, et al. Pediatric IBD-unclassified Is Less Common than Previously Reported; Results of an 8-Year Audit of the EUROKIDS Registry. Inflamm Bowel Dis 2015;21:2145-53. [Crossref] [PubMed]
- Ruel J, Ruane D, Mehandru S, et al. IBD across the age spectrum: is it the same disease? Nat Rev Gastroenterol Hepatol 2014;11:88-98. [Crossref] [PubMed]
- Hentze MW, Muckenthaler MU, Galy B, et al. Two to tango: regulation of Mammalian iron metabolism. Cell 2010;142:24-38. [Crossref] [PubMed]
- Daher R, Karim Z. Iron metabolism: State of the art. Transfus Clin Biol 2017;24:115-9. [Crossref] [PubMed]
- Camaschella C. Iron deficiency. Blood 2019;133:30-9. [Crossref] [PubMed]
- Cappellini MD, Comin-Colet J, de Francisco A, et al. Iron deficiency across chronic inflammatory conditions: International expert opinion on definition, diagnosis, and management. Am J Hematol 2017;92:1068-78. [Crossref] [PubMed]
- Peyrin-Biroulet L, Williet N, Cacoub P. Guidelines on the diagnosis and treatment of iron deficiency across indications: a systematic review. Am J Clin Nutr 2015;102:1585-94. [Crossref] [PubMed]
- Georgieff MK. Iron assessment to protect the developing brain. Am J Clin Nutr 2017;106:1588S-93S. [Crossref] [PubMed]
- Glance LG, Dick AW, Mukamel DB, et al. Association between intraoperative blood transfusion and mortality and morbidity in patients undergoing noncardiac surgery. Anesthesiology 2011;114:283-92. [Crossref] [PubMed]
- Peoc'h K. Saving patient blood: a new rule of life? Focus on pre-operative anemia. Ann Biol Clin (Paris) 2019;77:7-9. [Crossref] [PubMed]
- Langer AL, Ginzburg YZ. Role of hepcidin-ferroportin axis in the pathophysiology, diagnosis, and treatment of anemia of chronic inflammation. Hemodial Int 2017;21:S37-46. [Crossref] [PubMed]
- Ward DM, Kaplan J. Ferroportin-mediated iron transport: expression and regulation. Biochim Biophys Acta 2012;1823:1426-33. [Crossref] [PubMed]
- Peyrin-Biroulet L, Lopez A, Cummings JRF, et al. Review article: treating-to-target for inflammatory bowel disease-associated anaemia. Aliment Pharmacol Ther 2018;48:610-7. [Crossref] [PubMed]
- Bager P, Befrits R, Wikman O, et al. The prevalence of anemia and iron deficiency in IBD outpatients in Scandinavia. Scand J Gastroenterol 2011;46:304-9. [Crossref] [PubMed]
- Van Assche G, Dignass A, Bokemeyer B, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 3: special situations. J Crohns Colitis 2013;7:1-33. [Crossref] [PubMed]
- Stein PE, Badminton MN, Rees DC. Update review of the acute porphyrias. Br J Haematol 2017;176:527-38. [Crossref] [PubMed]
- González Alayón C, Pedrajas Crespo C, Marín Pedrosa S, et al. Prevalence of iron deficiency without anaemia in inflammatory bowel disease and impact on health-related quality of life. Gastroenterol Hepatol 2018;41:22-9. [PubMed]
- Basseri RJ, Nemeth E, Vassilaki ME, et al. Hepcidin is a key mediator of anemia of inflammation in Crohn's disease. J Crohns Colitis 2013;7:e286-91. [Crossref] [PubMed]
- Testa A, Rispo A, Romano M, et al. The burden of anaemia in patients with inflammatory bowel diseases. Dig Liver Dis 2016;48:267-70. [Crossref] [PubMed]
- Gisbert JP, Gomollón F. Common misconceptions in the diagnosis and management of anemia in inflammatory bowel disease. Am J Gastroenterol 2008;103:1299-307. [Crossref] [PubMed]
- Voegtlin M, Vavricka SR, Schoepfer AM, et al. Prevalence of anaemia in inflammatory bowel disease in Switzerland: a cross-sectional study in patients from private practices and university hospitals. J Crohns Colitis 2010;4:642-8. [Crossref] [PubMed]
- Lou DQ, Lesbordes JC, Nicolas G, et al. Iron- and inflammation-induced hepcidin gene expression in mice is not mediated by Kupffer cells in vivo. Hepatology 2005;41:1056-64. [Crossref] [PubMed]
- Nicolas G, Chauvet C, Viatte L, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest 2002;110:1037-44. [Crossref] [PubMed]
- Borren NZ, van der Woude CJ, Ananthakrishnan AN. Fatigue in IBD: epidemiology, pathophysiology and management. Nat Rev Gastroenterol Hepatol 2019;16:247-59. [Crossref] [PubMed]
- Dignass AU, Gasche C, Bettenworth D, et al. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohns Colitis 2015;9:211-22. [Crossref] [PubMed]
- Cacoub P, Vandewalle C, Peoc'h K. Using transferrin saturation as a diagnostic criterion for iron deficiency: A systematic review. Crit Rev Clin Lab Sci 2019;56:526-32. [Crossref] [PubMed]
- Wish JB. Assessing iron status: beyond serum ferritin and transferrin saturation. Clin J Am Soc Nephrol 2006;1:S4-8. [Crossref] [PubMed]
- Wang CY, Babitt JL. Hepcidin regulation in the anemia of inflammation. Curr Opin Hematol 2016;23:189-97. [Crossref] [PubMed]
- Poggiali E, Migone De Amicis M, Motta I. Anemia of chronic disease: a unique defect of iron recycling for many different chronic diseases. Eur J Intern Med 2014;25:12-7. [Crossref] [PubMed]
- Mast AE, Blinder MA, Gronowski AM, et al. Clinical utility of the soluble transferrin receptor and comparison with serum ferritin in several populations. Clin Chem 1998;44:45-51. [Crossref] [PubMed]
- Beguin Y. Soluble transferrin receptor for the evaluation of erythropoiesis and iron status. Clin Chim Acta 2003;329:9-22. [Crossref] [PubMed]
- Krawiec P, Pac-Kożuchowska E. Soluble transferrin receptor and soluble transferrin receptor/log ferritin index in diagnosis of iron deficiency anemia in pediatric inflammatory bowel disease. Dig Liver Dis 2019;51:352-7. [Crossref] [PubMed]
- Revel-Vilk S, Tamary H, Broide E, et al. Serum transferrin receptor in children and adolescents with inflammatory bowel disease. Eur J Pediatr 2000;159:585-9. [Crossref] [PubMed]
- Abitbol V, Borderie D, Polin V, et al. Diagnosis of Iron Deficiency in Inflammatory Bowel Disease by Transferrin Receptor-Ferritin Index. Medicine (Baltimore) 2015;94:e1011 [Crossref] [PubMed]
- Peoc'h K, Nicolas G, Schmitt C, et al. Regulation and tissue-specific expression of δ-aminolevulinic acid synthases in non-syndromic sideroblastic anemias and porphyrias. Mol Genet Metab 2019;128:190-7. [Crossref] [PubMed]
- Stein J, Dignass AU. Management of iron deficiency anemia in inflammatory bowel disease - a practical approach. Ann Gastroenterol 2013;26:104-13. [PubMed]
- Aksan A, Wohlrath M, Iqbal TH, et al. Inflammation, but Not the Underlying Disease or Its Location, Predicts Oral Iron Absorption Capacity in Patients With Inflammatory Bowel Disease. J Crohns Colitis 2020;14:316-22. [Crossref] [PubMed]
Cite this article as: Peoc’h K, Manceau H, Joly F, Treton X. Iron deficiency in chronic inflammatory bowel diseases: an update. J Lab Precis Med 2021;6:31.


