
Heart failure among patients with prediabetes and type 2 diabetes mellitus: diagnostic and predictive biomarkers: a narrative review
Introduction
Heart failure (HF) remains a serious public health and social-economic problem affecting more than 23 million patients worldwide (1). Acute HF and chronic HF with reduced (HFrEF) and mildly reduced (HFmrEF) left ventricular ejection fraction (HFrEF) remain a leading cause of cardiovascular (CV) mortality among in-patients including those who have clinically significant comorbidities including diabetes mellitus (DM) and chronic kidney disease (2). Dramatic increases in the number of new cases of DM worldwide have seen HF as a steadily growing life-threatening complication of that disease (3,4).
Several biomarkers are reported to be associated with HF, such as natriuretic peptides (NPs), high sensitive cardiac troponins, soluble suppressor of tumorigenesis-2 (sST2), and galectin-3 (5,6), and their diagnostic and predictive abilities substantially distinguish each other in HFrEF/HFmrEF and HFpEF patients, even those having co-morbidities. However, there is limited evidence to show their discriminative potency for DM progression and risk of HF development (7,8). Additionally, the economic burden of this strategy appears to be challenging and requires more attention before an optimal choice of biomarkers in routine clinical practice can be made.
The recently reported New Universal Definition and Classification of HF proports to use a biomarker strategy based on the measure of circulating and genetic indicators to identify patients at higher risk of HF (stage A) and pre-HF (stage B). This assists in determining the risk of moving these patients from early stages to the end stage of the disease, and to choose an optimal strategy to diagnose and treat it (9). Nevertheless, there is a large amount of conflicting data from research designed to distinguish the value of a biomarker strategy in HFpEF and HFrEF/HFmrEF (10-12). Although biomarkers can assist clinicians with timely diagnosis, risk stratification, and prognosis determination of HFrEF/HFmrEF and HFpEF patients to provide individualized treatment (13), there is no biomarker-guided strategy for DM patients at higher risk of HF and for HF patients with at risk or overt DM (14). This review provides an updated analysis of circulating biomarkers which can be used with an aim of improving diagnosis and prognosis among HF with DM. We present the following article in accordance with the Narrative Review reporting checklist (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-21-37/rc).
Methods and methodology
The MEDLINE, EMBASE, Medline (PubMed), Web of Science, and Cochrane Central databases were searched for English publications satisfying the following key words [heart failure]; [HFrEF]; [HFmrEF]; [HFpEF]; [diabetes mellitus], [type 2 diabetes mellitus], [pre-diabetes]; [pre-T2DM]; [cardiovascular risk], [cardiovascular risk factors], [cardiac biomarkers]; [circulating biomarkers]; [diagnosis]; and [prognosis]. All authors independently selected articles, evaluated the quality of the data, its presentation, and its interpretation correspondence to the main idea of the study, and constructed the final list of references.
Contemporary biomarker strategy to predict and diagnose HF
Biomarkers are promising surrogate indicators of pathologic changes in target organs (myocardium, lungs, kidney, vessels, adipose tissue, and skeletal muscles) and maladaptive homeostasis for patients with T2DM and different phenotypes of HF (15). Although biomarkers of biomechanical stress (NPs), myocardial injury (high sensitive cardiac troponins), fibrosis (sST2), and inflammation (galectin-3) have revealed variable results in their potency to predict HF onset at an early stage, diagnose HF, decrease the risk of admissions due to HF progression, and manage the condition, they undoubtedly remain a proof supporting personified therapy of HFrEF and HFpEF (16). Current clinical guidelines of the American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Failure Society of America (HFSA), and European Cardiology Society (ESC) have proposed the use of biomarkers in personalized medical care of HF patients regardless of T2DM, and to diagnose HF and stratify patients at higher risk of poor prognosis, despite some differences in recommendations for practical use (9,17). Table 1 reports the utilization of biomarkers in the diagnosis, prediction, and management of HF according to 2021 ESC and 2017 ACC/AHA clinical guidelines (9,17).
Table 1
| Recommendations | Biomarkers | COR | LOE | Phenotype of HF | Stage of HF |
|---|---|---|---|---|---|
| Prediction of HF | |||||
| ESC, 2021 | BNP/NT-proBNP | IIa | B | AHF, CHF | C, D |
| ACC/AHA/HFSA, 2017 | |||||
| Diagnosis of HF | |||||
| ESC, 2021 | BNP/NT-proBNP/MR-proANP | I | A | AHF, HFpEF, HFmrEF | A–C |
| ACC/AHA/HFSA, 2017 | I | A | AHF, CHF | ||
| Risk of in-hospital death | |||||
| ESC, 2021 | BNP/NT-proBNP | I | C | AHF | C, D |
| ACC/AHA/HFSA, 2017 | hs-cTn | I | C | AHF | |
| Risk of recurrent hospital admission | |||||
| ESC, 2021 | BNP/NT-proBNP | I | A | AHF, CHF | C, D |
| ACC/AHA/HFSA, 2017 | I | A | AHF, CHF | ||
| ESC, 2021 | hs-cTn | I | C | AHF, CHF | |
| ACC/AHA/HFSA, 2017 | I | IIa | AHF, CHF | ||
| ACC/AHA/HFSA, 2017 | Galectin-3 | IIb | B | AHF, CHF | |
| ACC/AHA/HFSA, 2017 | sST2 | IIb | B | AHF, CHF | C, D |
| Biomarker-guided therapy of HF | |||||
| ACC/AHA/HFSA, 2017 | BNP/NT-proBNP | I | A | HFrEF/HFmrEF/HFpEF | C |
ESC, European Cardiology Society; ACC, American College of Cardiology; AHA, American Heart Association; HFSA, Heart Failure Society of America; HF, heart failure; COR, class of recommendation; LOE, level of evidence; BNP, B-type natriuretic peptide; NT-proBNP, N-terminal pro-B-type natriuretic peptide; AHF, acute heart failure; CHF, chronic heart failure; MR-proANP, mid-regional pro A-type natriuretic peptide; HFpEF, heart failure preserved ejection fraction; HFrEF, heart failure reduced ejection fraction; hs-cTn, high sensitive cardiac troponins; sST2, soluble ST2; HFmrEF, heart failure with mildly reduced ejection fraction.
NPs
NPs are well known and deeply investigated indicators of biomechanical stress (18), which are gold standard biomarkers for HF with different phenotypes, as well as for acute/acutely decompensated HF (19). According to current clinical guidelines (9,17) B-type natriuretic peptide (BNP), N-terminal proBNP (NT-proBNP), and mid-regional-atrial NP (MR-proANP) are used for diagnosis and prognosis of HF (19), while their performance appears to be better for HFrEF and HFmrEF than for HFpEF (19,20). However, their role in risk stratification in high risk of HF development patients, including those who have DM (A stage of HF) and asymptomatic pre-HF individuals (B stage of HF), have further expanded beyond HF to several CV conditions, such as abdominal obesity, atrial fibrillation/flutter, T2DM, and systemic hypertension (21,22). While NPs are considered predictors for atrial fibrillation/flutter and HF manifestation in patients with DM and obesity, the serum levels of these biomarkers are remarkably variable for these patients and those who have chronic kidney disease (23,24). Adipose tissue accumulation was found to be associated with near normal plasma NP levels, but chronic kidney disease was, on the contrary, a frequent cause of a dramatic rise of NP levels due to lowered kidney clearance (25). In fact, patients with T2DM at risk of HF, CV events, and impaired renal function demonstrate different cut-off points to the diagnostic and predictive levels of MR-proANP, BNP, and NT-proBNP for acute and chronic HF (26). In this context, the optimum threshold values require adjustment to the estimated glomerular filtration ratio categories and body mass index in T2DM patients with a higher risk of HF and established HF. Indeed, the levels of NT-proBNP >400 pg/mL in patients with HFrEF who were included in the PARADIGM-HF trial (Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) and the ATMOSPHERE trial (Aliskiren Trial to Minimize Outcomes in Patients With Heart Failure) have demonstrated high predictive value for adverse CV outcomes, regardless of comorbidities such as atrial fibrillation (27). There is no point to argue that extremely high levels of NT-proBNP (>3,000 pg/mL) have optimal accuracy to diagnose acute/acutely decompensated HFrEF, while their ability in risk stratification and diagnostic utility among asymptomatic patients with HFpEF needs to be reappraised. Nevertheless, adding NT-proBNP to the traditional risk factor score significantly increased the predictive value of the whole model for major adverse CV events (MACEs) in pre-T2DM and T2DM patients (28).
In addition, among high CV risk patients (n=5,509) with T2DM who were enrolled in the ALTITUDE (Aliskiren in Type 2 Diabetes Using Cardiorenal Endpoints) trial, elevated levels of NT-proBNP predicted both CV mortality and composite CV events (CV death, resuscitated cardiac arrest, nonfatal myocardial infarction, stroke, or HF hospitalization) (29). Idzerda et al. (30) hypothesized that measure of the levels of NT-proBNP could predict the effects of additional therapy with aliskiren on cardio-renal endpoints among patients included in the ALTITUDE (Aliskiren in Type 2 Diabetes Using Cardiorenal Endpoints) trial. They reported the total number of cardio-renal endpoint events were reduced by 20% and 2% in the two lowest NT-proBNP tertiles respectively, among patients with T2DM treated with aliskiren. Similarly, CV and end-stage renal disease endpoints were substantially reduced in these individuals having lowers tertiles of NT-proBNP concentrations (30). The EXAMINE (Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care) trial, in which 5,380 patients with T2DM were included, has shown that dynamic NT-proBNP levels allowed re-stratifying risks for CV death and HF hospitalization (31).
The implementation of BNP biosensing platforms based on optical and electrochemical immunosensor methodology allowed sufficiently reducing total expenditures on BNP level monitoring in follow-up and demonstrated unsurpassed sensitivity, selectivity, and reproducibility (32). Prausmüller et al. (33) reported preliminary results of an investigation of the prognostic ability of the ESC/European Association for the Study of Diabetes (EASD) risk model compared to the Systematic COronary Risk Evaluation (SCORE) risk model and NT-proBNP among T2DM patients. The primary finding of the study was that the levels of NT-proBNP (0.80 versus 0.53, P<0.001) and SCORE (0.64 versus 0.53, P=0.001) had remarkably higher discriminative values than the ESC/EASD risk model for CV death and all-cause death (0.73, 0.66 versus 0.52, P<0.001 for both). Thus, single NT-proBNP plasma level measure would improve predicting 10-year CV disease and all-cause mortality in T2DM individuals.
The above results show NPs are the most accurate test for HF diagnosis and progression of the disease as well as for predicting adverse outcomes and guiding therapy.
Highly sensitive cardiac troponin (hs-cTn)
hs-cTn are biomarkers of myocardial injury and necrosis which are released from damaged cardiac myocytes into the circulation. hs-cTn are structural proteins of cardiac myocytes troponins commonly elevated in not only acute coronary syndrome/myocardial infarction, but also in clinical conditions that are strongly associated with subclinical myocardial damage due to biomechanical and oxidative stresses, inflammation, and volume overload (34). Nowadays hs-cTn T/I have been regarded as the gold-standard marker for cardiomyocyte necrosis having unprecedented predictive value for CV outcomes including all-cause and CV mortality, HF onset, hospitalization, and coronary revascularization (35).
In the last two decades, attempts have been made to identify minor asymptomatic myocardial injury and interpret its clinical relevance in patients beyond acute myocardial infarction and acute coronary syndromes. These attempts have been enabled by the development and use of new analytical approaches to detect cardiac troponins with higher accuracy and reproducibility. Indeed, the measurement of cardiac troponin levels using commercially available second generation high-sensitive tests has caused previous opinion of them as only indicators of myocardial injury to that of biomarkers of biochemical stress (36,37). Moreover, the increased risk for mortality, CV events, and HF-related complications correlated with high-sensitivity cardiac troponins even when their levels were mildly above the normal level (38). Yet, hs-cTnT/I elevation was found to be strongly linked to poor CV outcomes in several clinical conditions, even though the elevation was stable over time (39).
Mildly elevated levels of hs-cTnT/I have been shown in T2DM patients and individuals at risk of HF/established HF and independently predicted all-cause and CV mortality, hospitalization, and CV intervention (40-42). Interestingly, the ARIC (Atherosclerosis Risk in Communities) study showed that circulating levels of hs-cTnT among 3,056 adult patients with higher atherosclerotic risk were independently associated with diabetes status, while among participants without T2DM, there were also significant associations of NT-proBNP levels and the urine albumin-to-creatinine ratio (43). In addition, elevated levels of both hs-cTnI (≥9.4 ng/L) and hs-cTnT (≥25 ng/L) were remarkably associated with prevalent coronary heart disease, HF, chronic kidney disease, pulmonary disease, hypoglycemia, hypertension, dementia, and frailty (44). Yet, the CRIC Study showed that hsTnT levels in patients with established kidney impairment, including diabetes-induced chronic kidney disease, were associated with a greater risk for progression of the disease (45). A meta-analysis of 45 clinical studies showed that hs-cTnT had the highest sensitivity [0.86 (95% CI: 0.84–0.88)], specificity [0.82 (95% CI: 0.79–0.84)], positive predictive value [0.80 (95% CI: 0.77–0.83)], and negative predictive value [0.87 (95% CI: 0.85–0.89)] to diagnose HF compared with other biomarkers, such as copeptin, galectin-3, MR-proANP, MR-proadrenomedullin, and sST2 (46). In summary, hs-cTnT/I appears to be a general surrogate biomarker of myocardial damage and higher mortality risk in patients with T2DM and HF regardless of its phenotypes, while the powerful predictive value of hs-cTnT was found irrespective of comorbidity burden.
sST2
The novel biomarker of fibrosis and inflammation sST2, was included in the 2017 ACC/AHA/HFSA HF guideline, but not in the 2016 and 2021 ESC guidelines for HF as an alternative tool for HF prediction and risk stratification, although its diagnostic and discriminative utilities in T2DM require further elucidation (17). In addition, the 2021 ESC guidelines for HF recommend that more clinical evidence is needed before sST2 and other biomarkers as additional diagnostic tests should be included in the guidelines (17). However, unlike other inflammatory biomarkers, sST2 has been approved by experts from the ACC/AHA/HFSA for risk stratification of patients with HF, because it has demonstrated high accuracy and reproducibility in serial measures at a reasonable cost and improved predictive value of NPs and hs-cTnT/I for HF (14).
Being a member of the interleukin-1 receptor family, sST2 acts as an endogenous suppressor of beneficial impact of interleukin-33 on myocardium and vessels leading to cardiac hypertrophy, fibrosis, and dysfunction (47). Previous clinical studies have shown that elevated levels of sST2 demonstrated a high discriminative ability for predicting all-cause and CV mortality, sudden death, HF occurrence, HF-related events, and HF hospitalization regardless of common CV risk factors including T2DM, chronic kidney disease, and hypertension (48-51). Interestingly, in the comparative HF study, by Najjar et al. (52) patients with HFpEF had lower sST2 levels when compared to HFrEF individuals but were potentially more strongly related to poor outcomes.
There is evidence that the improved clinical status and hemodynamics in acutely decompensated HF patients were associated with a significantly greater decline in circulating sST2 levels (10), suggesting serial measures of sST2 levels could also stratify patients with HF at a higher risk of death (53,54). Furthermore, elevated levels of sST2 provide additional prognostic information for acute HF and different phenotypes of chronic HF, exceeding the ability of NT-proBNP (55). Thus, sST2 demonstrates powerful predictive value for clinical outcomes in HF patients regardless of T2DM presence, but high economic burden is considered as the main constraint for an implementation of single and serial sST2 measures in routine clinical practice (56).
Galectin-3
Galectin-3 is a multiphase protein belonging to the β-galactoside-binding lectin family (57) that is overexpressed in different types of cells due to tissue injury or stress (58). There has been a continuously rising interest in galectin-3 due to extensive studies involving the molecule in adverse cardiac remodeling, atherosclerosis, and T2DM. Indeed, over-expression of galectin-3 in the myocardium increased collagen I protein production and fibronection accumulation via the protein kinase C-α pathway leading to myocardial fibrosis and hypertrophy (59). In addition, experimental data raised a hypothesis that the detrimental proliferative effect of aldosterone might be at least particularly mediated by galectin-3 (60,61), suggesting that in this context, galectin-3 is both a biomarker and causal factor for HFpEF and HFrEF/HFmrEF (61).
In clinical settings galectin-3 was found to be a better predictor for HFpEF than HFrEF/HFmrEF (62). The observational Diast-CHF study that enrolled 1,386 patients at high risk of HF or with suspected HF showed that galectin-3 being added to NT-proBNP significantly improved predictive value for the combined model to diagnose HFpEF (63). Although the admission levels of galectin-3 among in-patients with HFrEF were strongly correlated with higher interleukin-6 and C-reactive protein levels and independently associated with all-cause mortality and HF hospitalization, serial measures of galectin-3 levels over 6 months did not improve prognostic value compared with baseline concentrations (64). de Boer et al. (65) investigated the associations of 12 CV biomarkers (hs-cTnT or I, C-reactive protein, urinary albumin to creatinine ratio, renin to aldosterone ratio, D-dimer, fibrinogen, sST2, galectin-3, cystatin C, plasminogen activator inhibitor 1, and interleukin-6) with incident HFpEF versus HFrEF among adults from the general population after an adjustment for CV risk factors, and did not find a direct relation between galectin-3 and certain phenotypes of chronic HF. In contrast, Kanukurti et al. (66) reported that elevated levels of galectin-3 were the most optimal predictive biomarker for HFpEF manifestation.
Considering T2DM patients and metabolic syndrome individuals are at a higher risk of HFpEF than HFrEF, galectin-3 might ideally lead to a reclassification of cardiometabolic risk. The Dallas Heart Study showed that levels of galectin-3 correlated well with incident T2DM, metabolic syndrome, and body fat compartments (67) and positively correlated with levels of hs-CRP, IL-18, monocyte chemoattractant protein 1, soluble TNF receptor 1A, myeloperoxidase, C-peptide, and homeostatic model assessment for insulin resistance (68). Overall, cross-sectional analyses of 2,946 Framingham Heart Study participants unveiled that circulating levels of galectin-3 were associated with higher body mass index, waist circumference, hypertension, and triglycerides levels (68). Despite investigators emphasizing that galectin-3 levels being adjusted to cardiometabolic risk factors were not able to predict incident cardiometabolic disease, it remained a powerful predictor for T2DM and metabolic syndrome in the general population.
Alternative biomarkers
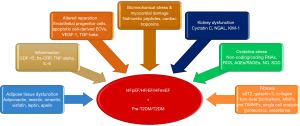
Although a conventional biomarker strategy based mainly on NPs and cardiac troponins is recommended to diagnose any phenotype of HF, its predictive ability appears to be higher for HFrEF, whereas a risk stratification of patients with suspected or overt HFpEF needs improvement (69). In this context, previous investigations have revealed that biomarkers of biomechanical stress, cardiac injury and necrosis, and inflammation markedly better predict HFrEF than HFpEF (14,21). Consequently, biomarkers of fibrosis, oxidative stress, adipose tissue dysfunction, and altered endogenous reparation are increasingly being used to assess HFpEF and probably HFmrEF (42,56). Figure 1 illustrates a network of conventional and alternative biomarkers in HF patients having pre-diabetes (pre-DM) or T2DM, and the most informative of these are discussed in the section below.
Biomarkers of oxidative stress
The role of biomarkers reflecting oxidative stress is controversial (70). In fact, T2DM modulates the expression of the NFE2L2 gene that essentially encodes the key transcription factor Nrf2 and regulates the expression of antioxidant and detoxification genes (71,72). In addition, Nrf2 is activated in various CV diseases including HF, and supports cardiac protection through regulation of genes that are involved in cell signaling, differentiation, transcription, proliferation, energy metabolism, and autophagy (73). Therefore, oxidative stress enhances mitochondrial damage and contributes to cell injury, extracellular matrix re-modeling, and altered tissue reparation (74). Although subclinical oxidative stress and inflammatory conditions are the result of HF comorbidities including T2DM with abdominal obesity, there is no strong evidence that conventional circulating biomarkers of oxidative stress, such as superoxide dismutase (SOD), reactive oxygen species (ROS), peroxide species of lipids, and AGE/RAGEs provide additional prognostic information for HF patients with T2DM (75,76). Future investigations may evaluate non-coding RNAs analysis and proteomics/secretom of extracellular vesicles (ECVs) (77). Non-coding RNAs, including small non-coding RNAs (microRNAs, circular RNAs, and long non-coding RNAs), as regulators of insulin resistance, cardiomyocytes apoptosis, microvascular inflammation, and myocardial hypertrophy, are of interest, especially due to their protective effect on cardiac function in HF patients (78,79). The testing of oxidative stress biomarkers needs to be investigated in future clinical interventions to evaluate their potential role in stratification of T2DM patients at high risk of CVD and HF occurrence.
Biomarkers of adipose tissue dysfunction
The development of HF in T2DM patients closely relates to comorbidities such as abdominal obesity, diabetes-induced kidney disease, and myocardial infarction (79). Ectopic perivascular and pericardial adipose tissue, along with other white adipose tissue (WAT), are the source of synthesis and release of active molecules called adipokines that exhibit pro-inflammatory as well as anti-inflammatory properties with the capacity to directly affect the energy metabolism of myocardium and skeletal muscles, vascular integrity, endothelial function, and insulin resistance (80). There is a large body of evidence regarding the association of exacerbated WAT inflammation with the altered circulating profile of adipokines (81,82). Indeed, HF patients have lowered levels of omentin, zinc-α2-glycoprotein, glypican-4, apelin, and chemerin, and increased levels of adiponectin, resistin, and leptin (83-85).
The results of the Framingham Offspring Study have shown that an incident HF might accompany increased circulating levels of several adipokines, such as resistin, and that this relationship was changed after adjustment for prevalent CAD, abdominal obesity, insulin resistance, and inflammation (86). In contrast, adiponectin did not show a significant association with the risk of HF (84). Therefore, adverse cardiac remodeling in T2DM patients is associated with altered adiponectin/leptin ratio (86-88). Among the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam cohort the levels of chemerin-1 and omentin were found to be powerful predictors for atherosclerotic CVD, whereas chemerin-1, but not omentin, yielded discriminative potency for incident HF (89). Overall, the relationships between serum levels of the majority of pro-inflammatory adipokines and CV risk and a risk of HF development were not linear but J-shaped (adiponectin) or U-shaped (chemerin-1, omentin) associations, which were diminished for adiponectin after adjustment for additional potential confounders (88,89). However, decreased levels of apelin, chemerin-1, omentin-1, visfatin, and increased levels of adiponectin and leptin were found to have predictive value for CV mortality and HF progression in both T2DN and non-T2DM patients with overt HF (90-95). Thus, an altered profile of adipokines can be a novel circulating biomarker for predicting poor CV and HF-related events in patients with HF regardless of the presence of T2DM.
Biomarkers of calcium and phosphate metabolism
Altered calcium and phosphate metabolism plays an important role in the development of T2DM-dependent angiopathy, ectopic vascular calcification, and adverse cardiac remodeling. Blood level changes in several circulating biomarkers, including osteonectin, osteoprotegerin, osteopontin, and RANK ligand correspond well to risk progression of T2DM and HFpEF and CV mortality. One of the most promising of these is fetuin-A.
Fetuin-A
Fetuin-A (also known as alpha2-Heremans-Schmid glycoprotein), is a protein secreted from hepatocytes (so-called hepatokine) and is known to exert multiple physiological and pathophysiological functions including the inhibition of calcification processes and protein transport for calcium and phosphate. Previous studies have shown that low fetuin-A levels were associated with increased CV mortality (96). Moreover, it can interact with insulin receptors and is associated with the development of metabolic syndrome and progressive atherosclerotic disease. High levels of fetuin-A are thought to have protective effects in inflammatory conditions (e.g., in adipose tissue inflammation), while associations of low fetuin-A concentrations have been described with coronary artery disease and valvular calcification. Lower levels of fetuin-A have also been found in ischemic cardiomyopathy in comparison to dilative cardiomyopathy. This led to the assumption that fetuin-A might serve as a potential discriminator or biomarker for differentiation between those two disease entities (97).
Of special interest is the role of fetuin-A in the development of diabetes, as it is an inhibitor of the insulin receptor tyrosine kinase in diverse tissues (98,99), and both animal and clinical studies have shown that fetuin-A effects insulin resistance. Whereas high levels have been associated with lower rates of vascular calcification, in the context of diabetes accompanied with insulin resistance and obesity, more negative effects of high fetuin-A concentrations have been found (100,101). For example, patients with T2DM evidenced elevated fetuin-A levels in comparison to those without diabetes (102-104). This phenomenon has been verified by three meta-analyses (105-107).
These different regulatory mechanisms in patients with diabetes compared to cardio-vascular patients without diabetes remain poorly understood. The answer to this conundrum might be the fact that fetuin-A exerts an inhibitory signal to the insulin receptor tyrosine kinase which leads to a reduction in phosphorylation of the insulin receptor (108,109). A secondary effect might also be of interest as fetuin-A can aggravate insulin resistance via toll-like receptor 4, subsequently affecting adipose tissue inflammation and finally increasing resistance to insulin signaling (108). This pathway might also be influenced by an altered expression of adiponectin with its anti-inflammatory effects and functions on insulin-sensitization (110). Still, the pathophysiological connection between fetuin-A and T2DM-indiced HF is not fully elucidated.
Bone-related proteins
Bone-related proteins (osteopontin, osteoprotegerin, osteonectin, tenascin C, and thrombospondins 1 and 2) are matricellular proteins involved in the modulators of bone development, cardiac and vascular remodeling, and tissue regeneration (111). Previous studies showed that several members of the bone-related proteins family, such as tenascin-C, osteopontin, and osteonectin were up-regulated after ischemic myocardial injury and inflammation (112). However, a growing body of evidence strongly demonstrates that both inflammatory and reparative processes are under close regulation of bone-related proteins, which are released in response to several stimuli, such as ischemia/hypoxia, inflammation, and biomechanical stress, and provide tissue protective capacity (113).
An increased expression of osteopontin, an extracellular matrix protein, is known to lead to hypertrophy of the myocardium and the development of HF (114). In the diabetic heart, osteopontin shows higher expression in response to high glucose levels by signaling via angiotensin II and protein kinase C, and both cardiomyocytes and cardiac fibroblasts respond to higher glucose concentrations with osteopontin expression. Elevated levels of osteopontin were associated with an increased CV risk in T2DM patients, whereas other bone related proteins were not (115,116). Its expression increased greatly after myocardial ischemia, and data obtained in transgenic mouse studies suggests that osteopontin has some protective effects in myocardial remodeling after infarction via a modulation of collagen production and fibrosis (114). In the setting of HF, levels of osteopontin increase in concordance with the severity of HF, and its regulation of myocardial remodeling has been shown a potentiation of galectin-3 up-regulation and secretion (115). In addition, osteopontin improved HF diagnosis when combined with NT-proBNP. From a diagnostic point of view, osteopontin can provide help for assessing acute HF (115,116), and in in patients suffering from HFpEF, it has been shown to be of prognostic potential in a multi-marker analysis (115). Moreover, elevated levels of osteopontin predicted high 1- and 5-year CV mortality and re-hospitalization due to HF (117,118). Reports have confirmed the predictive role of other bone-related proteins for CV events and HF outcomes in studies with higher proportions of patients with chronic kidney disease or large coronary artery occlusive disease (116-120). Cumulatively, bone-related proteins as markers of CV risk on diabetics with HF seem to be promising biomarkers, but more clinical trials are required to elucidate their role.
Growth differential factor-15 (GDF15)
GDF15 is a stress-induced multifactorial cytokine which belongs to the transforming growth factor (TGF) beta superfamily and is markedly expressed in a wide range of cells in both normal and pathological conditions (119). Acting as a suppressor of JNK, Bcl-2-associated death promoter (Bad), and epidermal growth factor receptor (EGFR) and activator of Smad/eNOS, PI3K/AKT signaling pathways GDF-15 improves glucose and energy homoeostasis, regulates appetite, potentiates weight loss, and induces tissue protection from ischemia/oxidative stress damage (120,121). Importantly, GDF15 was found to be a crucial mediator of anorexia-cachexia syndrome in advanced stages of severe HF, T2DM, nonalcoholic fatty liver disease, chronic renal disease, and cancer (122).
The clinical relevance of GDF-15 in energy homeostasis was thoroughly established in the XENDOS (XENical in the prevention of Diabetes in Obese subjects) trial that included 496 obese, nondiabetic individuals (123). Investigators found that GDF15 levels were strongly associated with BMI, waist-to-hip ratio, and insulin resistance (123). Echouffo-Tcheugui et al. (124) also established that higher circulating levels of GDF15 were positively associated with (highest versus lowest quartile) occurrence of T2DM [adjusted odds ratio (aOR) =2.48; 95% CI: 1.89–3.26], HF (aOR =3.22; 95% CI: 2.13–4.85), atherosclerotic CV events (aOR =1.57; 95% CI: 1.16–2.11), elevated levels of hs-cTnT (aOR =2.27; 95% CI: 1.54–3.34), and NT-proBNP (aOR =1.98; 95% CI: 1.46–2.70) in the general population.
Among HFpEF patients included in the multicenter PROMIS-HFpEF (Prevalence of Microvascular Dysfunction in Heart Failure With Preserved Ejection Fraction) study, elevated levels of GDF15 mediated the relationship between metabolic comorbidity and echocardiographic parameters, such as mitral E velocity, E/e' ratio, and tricuspid regurgitation velocity (125). A pooled analysis of both cohorts of patients who were enrolled in the PIVUS (Prospective Investigation of the Vasculature in Uppsala Seniors) study (n=901) and the ULSAM (Uppsala Longitudinal Study of Adult Men) study (n=685) unveiled that elevated levels of GDF15 were better associated with worsened left ventricular systolic function, but not diastolic dysfunction (50). Kanagala et al. (126) reported that both focal and diffuse fibrosis of the myocardium corresponded to increased GFD15 levels, while GDF15 and composite event (all-cause mortality and/or HF hospitalization) rates did not distinguish HFrEF and HFpEF patients. However, there is a large body of evidence regarding the possibility that GDF15 may improve prognostic information in terms of predominantly HFrEF being added to the NYHA functional class, LVEF, and serum levels of NT-proBNP (127,128). A recent systematic review by Rabkin and Tang (129) has shown that to distinguish HFpEF from HFrEF, GDF15, along with other inflammatory biomarkers, might be incorporated into a conventional biomarker strategy. Yet, Bouabdallaoui et al. (130) reported that GDF-15 was not significantly modified by ARNI sacubitril/valsartan among out-patients with HFrEF, while the baseline levels of this biomarker were strongly associated with all-cause mortality and CV outcomes (130). In summary, GDF-15 is considered a promising indicator of poor clinical outcomes in HFrEF/HFpEF and a predictor of the occurrence of HFpEF rather than HFrEF.
Biomarkers of kidney injury and dysfunction
Circulating biomarkers of acute kidney injury (AKI) and renal dysfunction, such as cystatin C, neutrophil gelatinase-associated lipocalin (NGAL), and kidney injury molecule-1 (KIM-1), were associated with CV events and were significantly higher in decedents with HF than in survivors with HF. However, research evaluating relationships between serum concentrations of these biomarkers and AKI, CV risk factors, BNP, NT-proBNP, circulating biomarkers of collagen homeostasis, HFpEF occurrence, HF progression, HF NYHA functional class, and mortality shows disparate results (131-134). For instance, increased cystatin C levels in serial measurements were not accurate for predicting AKI in HF but remained independently associated with mortality (132). A meta-analysis of 10 randomized clinical trials by Chen et al. (132) showed an elevated cystatin C level was positively associated with an increased risk of all-cause mortality and re-admission due to HF progression regardless of creatinine and estimated glomerular filtration rate (eGFR). However, cystatin C was not able to predict CV events and CV disease in patients with CV risk factors and T2DM (133,134).
In contrast to cystatin C, elevated levels of NGAL accurately predicted acute renal dysfunction in patients with chronic HF regardless of eGFR (135). In addition, NGAL correlated positively with galectin-3 in HF patients (136), as well as with cardiac hypertrophy and diastolic dysfunction in T2DM individuals (137). Data received from the Farmacology and NeuroHumoraL activation (FAR NHL) multicenter prospective registry have shown that serum levels of NGAL (>80 ng/mL) were a stronger predictor of 1-year all-cause mortality, acute HF hospitalization, left ventricle assist device implantation, and orthotopic heart transplantation (126). The multicenter, prospective GALLANT (NGAL EvaLuation Along with B-type NaTriuretic Peptide in acutely Decompensated Heart Failure) study revealed that elevated levels of NGAL along with a high BNP value at the time of discharge were strong predictors for 30-day out-comes in patients admitted for acute HF (137). Therefore, NGAL has been identified as a prospective biomarker for the management of acute HF, but not chronic HF (138). When included in multiple biomarker models, NGAL gave additive predictive value for incident HF, but it was no longer associated with mortality (139-142).
Serum levels of KIM-1 were found to be elevated in patients with T2DM-induced kidney disease and HF and correlated with eGFR and NGAL, but not with CV risk factors or the albumin-to-creatinine ratio (142,143). Previous studies showed no association of elevated serum levels of KIM-1 with clinical outcome in either acute or chronic HF after adjustment for NT-proBNP but predicted re-hospitalization in patients with acute HF (144-146). In fact, KIM-1 can improve the discriminative potency of CV risk factors for prediction of HF occurrence, whereas its ability to add prognostic information to conventional scores in patients with overt HF remains uncertain.
Biomarkers of altered cardiac and vascular reparation
Endothelial (EPC) and mesenchymal (MPC) progenitor cells are a core element of the endogenous reparation system, which plays a pivotal role in restoring architecture of the myocardium and extracellular matrix, vascular integrity, and endothelial and cardiac function after injury (147). Progenitor cells are involved in neovascularization, angiogenesis, reendothelization, and tissue reparation through the enhancement of cell proliferation, differentiation, and survival (148). There is a large amount of evidence that low numbers and weak function of EPCs/MPCs are independent predictors of adverse cardiac remodeling and poor clinical outcomes including death and hospitalization in patients with overt CV disease, T2DM, and HF (149-152). Therefore, circulating EPCs/MPCs could not only be powerful predictive biomarkers for the occurrence of HFpEF and progression of HFrEF, but also markers to assess multiple therapeutic strategies directed to the attenuation of adverse cardiac remodeling, muscle myopathy, and vascular function in HF (153-155). Although the number and function of both EPCs and MPCs appear to be powerful predictors for HF and T2DM-related complications, it is not clear whether these new biomarkers add additional predictive information to conventional models based on CV risk factors, phenotypes of HF, and traditional biomarkers.
Multiple biomarker strategies
Multiple biomarker predictive models are considered an effective method to increase specificity and sensitivity of a single biomarker tool (5). Data confirm the superiority of multiple models compared with conventional models in risk stratification in HFpEF, whereas the adoption of biomarker serial measurements for risk stratification in HFpEF remains uncertain. Different combinations of circulating cardiac biomarkers are likely a promising tool to improve prediction, risk stratification, and therapy in T2DM with HF, although there is limited data on the optimal number of biomarkers that can be allocated to improve point-of-care therapy among both HFrEF and HFpEF patients (156). There is no strong evidence that single biomarker use is superior to a multiple biomarker strategy for every clinical condition in HF patients. For instance, the MOLITOR (Impact of Therapy Optimisation on the Level of Biomarkers in Patients with Acute and Decompensated Chronic Heart Failure) study has shown that serial measurements of multiple biomarkers (C-terminal fragment of pre-pro-vasopressin, NT-proBNP, mid-regional pro-atrial NP, mid-regional pro-adrenomedullin, and C-terminal pro-endothelin-1) in advanced HF were no better than measurement of C-terminal fragment of pre-pro-vasopressin (157). Pandey et al. (158) evaluated the application of a biomarker-based risk score to identify patients with dysglycemia who were at high risk for incident HF. By enrolling individuals from three cohort studies; [ARIC, DHS, and Multi-Ethnic Study of Atherosclerosis (MESA)], the original biomarker score included hs-cTnT ≥6 ng/L, NT-proBNP ≥125 pg/mL, hs-C-reactive protein (hs-CRP) ≥3 mg/L, and left ventricular hypertrophy identified by electrocardiography with one point for each abnormal parameter. The authors found that the 5-year risk for HF was associated with an increase in biomarker score, and the highest risk was seen in patients with total scores of ≥3 (diabetes: 12.0%; pre-DM: 7.8%), showing biomarker scores could stratify HF risk among patients with T2DM and pre-DM. Berezin et al. (159) reported that the combination of NT-proBNP and sST2 had higher prognostic ability when compared with each biomarker alone in patients with acute HF, except for galectin-3 and hs-CRP, which did not increase in discriminative potency when compared to a multiple biomarker model in ischemia-induced HF. However, a number of circulating CD31+/annexin V+ ECVs and EPCs improved the predictive ability for conventional HF biomarkers (NPs, sST2, galectin-3) (160,161). Therefore, while several novel biomarkers such as sST2 and GDF-15, and fetuin A, correlated to each other and conventional biomarkers (NT-proBNP and high sensitive cardiac troponins), these correlations were not found in connection with an increased risk of HF development (161). In addition, biomarkers reflecting myocardial fibrosis and inflammation, such as galectin-3, N-terminal pro-peptide of procollagen type III, and sST2, marginally improved the predictive ability of conventional models for adverse cardiac remodeling and dysfunction (162). Consequently, these conflicting results deserve closer investigation in large clinical trials in the future.
Limitations
The are several limitations to this review which include the small number of face-to-face comparative investigations of circulating biomarkers in specifically designed large clinical trials, especially studies depicting NPs and cardiac troponins. In addition, the results of cohort studies dedicated to alternative biomarkers, are mainly based on small sample size, although their quality is quite high. However, although not desirable, we believe these limitations do not compromise the accuracy of the reported findings or lead to their misinterpretation.
Conclusions
Patients with pre-DM/T2DM frequently have HFpEF, rather than HFrEF/HFmrEF, and need an improved biomarker strategy for risk stratification and prognosis of the disease. Conventional biomarkers, such as NPs and cardiac troponins have an optimal ability to diagnose and predict HFrEF and HFpEF, and are recommended by current guidelines on HF. However, their utility to stratify individuals at risk and manage patients with HFpEF is limited due to their high variability in the presence of CV disease and metabolic comorbidities. Alternative biomarkers reflecting several pathological stages of HF progression (inflammation, endothelial dysfunction, oxidative stress, altered vascular and myocardial reparation, adipose tissue dysfunction, and skeletal muscle metabolism) continue to be investigated as new powerful tools to improve the discriminative power of traditional predictive scores. Large clinical studies are required to better elucidate whether a multiple biomarker approach including both conventional and alternative biomarkers will be clinically useful and cost effective.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-21-37/rc
Peer Review File: Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-21-37/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-21-37/coif). AEB serves as an unpaid editorial board member of Journal of Laboratory and Precision Medicine from July 2020 to July 2022. ML received Lecture fees for Johnson and Johnson and Daiichi Sanky, and was supported by Bayer AG for attending meetings. But all these do not have relevant conflict of interest in regards of the manuscript. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Virani SS, Alonso A, Aparicio HJ, et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021;143:e254-743. [Crossref] [PubMed]
- Murphy SP, Ibrahim NE, Januzzi JL Jr. Heart Failure With Reduced Ejection Fraction: A Review. JAMA 2020;324:488-504. [Crossref] [PubMed]
- Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 2018;14:88-98. [Crossref] [PubMed]
- Orso F, Fabbri G, Maggioni AP. Epidemiology of Heart Failure. Handb Exp Pharmacol 2017;243:15-33. [Crossref] [PubMed]
- Topf A, Mirna M, Ohnewein B, et al. The Diagnostic and Therapeutic Value of Multimarker Analysis in Heart Failure. An Approach to Biomarker-Targeted Therapy. Front Cardiovasc Med 2020;7:579567. [Crossref] [PubMed]
- Berezin AE. Circulating Biomarkers in Heart Failure. Adv Exp Med Biol 2018;1067:89-108. [Crossref] [PubMed]
- Abbasi A, Sahlqvist AS, Lotta L, et al. A Systematic Review of Biomarkers and Risk of Incident Type 2 Diabetes: An Overview of Epidemiological, Prediction and Aetiological Research Literature. PLoS One 2016;11:e0163721. [Crossref] [PubMed]
- Laakso M. Biomarkers for type 2 diabetes. Mol Metab 2019;27S:S139-46. [Crossref] [PubMed]
- Bozkurt B, Coats A, Tsutsui H. Universal Definition and Classification of Heart Failure. J Card Fail 2021:S1071-9164(21)00050-6.
- Morrow DA, Velazquez EJ, DeVore AD, et al. Cardiovascular biomarkers in patients with acute decompensated heart failure randomized to sacubitril-valsartan or enalapril in the PIONEER-HF trial. Eur Heart J 2019;40:3345-52. [Crossref] [PubMed]
- Patel RB, Vaduganathan M, Felker GM, et al. Physical Activity, Quality of Life, and Biomarkers in Atrial Fibrillation and Heart Failure With Preserved Ejection Fraction (from the NEAT-HFpEF Trial). Am J Cardiol 2019;123:1660-6. [Crossref] [PubMed]
- Chirinos JA, Orlenko A, Zhao L, et al. Multiple Plasma Biomarkers for Risk Stratification in Patients With Heart Failure and Preserved Ejection Fraction. J Am Coll Cardiol 2020;75:1281-95. [Crossref] [PubMed]
- Israr MZ, Heaney LM, Suzuki T. Proteomic Biomarkers of Heart Failure. Heart Fail Clin 2018;14:93-107. [Crossref] [PubMed]
- Chow SL, Maisel AS, Anand I, et al. Role of Biomarkers for the Prevention, Assessment, and Management of Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2017;135:e1054-91. [Crossref] [PubMed]
- Gaggin HK, Januzzi JL Jr. Biomarkers and diagnostics in heart failure. Biochim Biophys Acta 2013;1832:2442-50. [Crossref] [PubMed]
- Emani S. Remote Monitoring to Reduce Heart Failure Readmissions. Curr Heart Fail Rep 2017;14:40-7. [Crossref] [PubMed]
- Jay R, Jung SB, Park BH, et al. Compensatory structural and functional adaptation after radical nephrectomy for renal cell carcinoma according to preoperative stage of chronic kidney disease. Choi DK, Jung SB, Park BH, Jeong BC, Seo SI, Jeon SS, Lee HM, Choi HY, Jeon HG. J Urol. 2015 Oct;194(4):910-5. Epub 2015 Apr 28. doi:
10.1016/j.juro.2015.04.093 . Urol Oncol 2017;35:118-9.10.1016/j.juro.2015.04.093 - Nakagawa Y, Nishikimi T, Kuwahara K. Atrial and brain natriuretic peptides: Hormones secreted from the heart. Peptides 2019;111:18-25. [Crossref] [PubMed]
- Maisel AS, Duran JM, Wettersten N. Natriuretic Peptides in Heart Failure: Atrial and B-type Natriuretic Peptides. Heart Fail Clin 2018;14:13-25. [Crossref] [PubMed]
- Pieske B, Tschöpe C, de Boer RA, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 2019;40:3297-317. [Crossref] [PubMed]
- Goetze JP, Bruneau BG, Ramos HR, et al. Cardiac natriuretic peptides. Nat Rev Cardiol 2020;17:698-717. [Crossref] [PubMed]
- Oikonomou E, Zografos T, Papamikroulis GA, et al. Biomarkers in Atrial Fibrillation and Heart Failure. Curr Med Chem 2019;26:873-87. [Crossref] [PubMed]
- Volpe M, Battistoni A, Rubattu S. Natriuretic peptides in heart failure: Current achievements and future perspectives. Int J Cardiol 2019;281:186-9. [Crossref] [PubMed]
- Pagel-Langenickel I. Evolving Role of Natriuretic Peptides from Diagnostic Tool to Therapeutic Modality. Adv Exp Med Biol 2018;1067:109-31. [Crossref] [PubMed]
- Wolsk E, Claggett B, Pfeffer MA, et al. Role of B-Type Natriuretic Peptide and N-Terminal Prohormone BNP as Predictors of Cardiovascular Morbidity and Mortality in Patients With a Recent Coronary Event and Type 2 Diabetes Mellitus. J Am Heart Assoc 2017;6:004743. [Crossref] [PubMed]
- Chenevier-Gobeaux C, Guerin S, André S, et al. Midregional pro-atrial natriuretic peptide for the diagnosis of cardiac-related dyspnea according to renal function in the emergency department: a comparison with B-type natriuretic peptide (BNP) and N-terminal proBNP. Clin Chem 2010;56:1708-17. [Crossref] [PubMed]
- Kristensen SL, Jhund PS, Mogensen UM, et al. Prognostic Value of N-Terminal Pro-B-Type Natriuretic Peptide Levels in Heart Failure Patients With and Without Atrial Fibrillation. Circ Heart Fail 2017;10:e004409. [Crossref] [PubMed]
- Liu HH, Cao YX, Jin JL, et al. Prognostic value of NT-proBNP in patients with chronic coronary syndrome and normal left ventricular systolic function according to glucose status: a prospective cohort study. Cardiovasc Diabetol 2021;20:84. [Crossref] [PubMed]
- Malachias MVB, Jhund PS, Claggett BL, et al. NT-proBNP by Itself Predicts Death and Cardiovascular Events in High-Risk Patients With Type 2 Diabetes Mellitus. J Am Heart Assoc 2020;9:e017462. [Crossref] [PubMed]
- Idzerda NMA, Persson F, Pena MJ, et al. N-terminal pro-brain natriuretic peptide (NT-proBNP) predicts the cardio-renal response to aliskiren in patients with type 2 diabetes at high renal and cardiovascular risk. Diabetes Obes Metab 2018;20:2899-904. [Crossref] [PubMed]
- Sharma A, Vaduganathan M, Ferreira JP, et al. Clinical and Biomarker Predictors of Expanded Heart Failure Outcomes in Patients With Type 2 Diabetes Mellitus After a Recent Acute Coronary Syndrome: Insights From the EXAMINE Trial. J Am Heart Assoc 2020;9:e012797. [Crossref] [PubMed]
- Alawieh H, Chemaly TE, Alam S, et al. Towards Point-of-Care Heart Failure Diagnostic Platforms: BNP and NT-proBNP Biosensors. Sensors (Basel) 2019;19:5003. [Crossref] [PubMed]
- Prausmüller S, Resl M, Arfsten H, et al. Performance of the recommended ESC/EASD cardiovascular risk stratification model in comparison to SCORE and NT-proBNP as a single biomarker for risk prediction in type 2 diabetes mellitus. Cardiovasc Diabetol 2021;20:34. [Crossref] [PubMed]
- Park KC, Gaze DC, Collinson PO, et al. Cardiac troponins: from myocardial infarction to chronic disease. Cardiovasc Res 2017;113:1708-18. [Crossref] [PubMed]
- Passino C, Aimo A, Masotti S, et al. Cardiac troponins as biomarkers for cardiac disease. Biomark Med 2019;13:325-30. [Crossref] [PubMed]
- Berezin AE. Biomarkers in Heart Failure. J Blood Lymph 2017;7:172-9.
- Holzmann MJ. Clinical implications of high-sensitivity cardiac troponins. J Intern Med 2018;284:50-60. [Crossref] [PubMed]
- Lippi G, Sanchis-Gomar F. "Ultra-sensitive" cardiac troponins: Requirements for effective implementation in clinical practice. Biochem Med (Zagreb) 2018;28:030501. [Crossref] [PubMed]
- Hammarsten O, Mair J, Möckel M, et al. Possible mechanisms behind cardiac troponin elevations. Biomarkers 2018;23:725-34. [Crossref] [PubMed]
- Tang O, Matsushita K, Coresh J, et al. High-Sensitivity Cardiac Troponin I and T for Cardiovascular Risk Stratification in Adults With Diabetes. Diabetes Care 2020;43:e144-6. [Crossref] [PubMed]
- Gidding SS, Bacha F, Bjornstad P, et al. Cardiac Biomarkers in Youth with Type 2 Diabetes Mellitus: Results from the TODAY Study. J Pediatr 2018;192:86-92.e5. [Crossref] [PubMed]
- Berezin AE, Berezin AA. Circulating Cardiac Biomarkers in Diabetes Mellitus: A New Dawn for Risk Stratification-A Narrative Review. Diabetes Ther 2020;11:1271-91. [Crossref] [PubMed]
- Hicks CW, Wang D, Daya NR, et al. Associations of Cardiac, Kidney, and Diabetes Biomarkers With Peripheral Neuropathy among Older Adults in the Atherosclerosis Risk in Communities (ARIC) Study. Clin Chem 2020;66:686-96. [Crossref] [PubMed]
- Tang O, Daya N, Matsushita K, et al. Performance of High-Sensitivity Cardiac Troponin Assays to Reflect Comorbidity Burden and Improve Mortality Risk Stratification in Older Adults With Diabetes. Diabetes Care 2020;43:1200-8. [Crossref] [PubMed]
- Bansal N, Zelnick L, Shlipak MG, et al. Cardiac and Stress Biomarkers and Chronic Kidney Disease Progression: The CRIC Study. Clin Chem 2019;65:1448-57. [Crossref] [PubMed]
- Huang Z, Zhong J, Ling Y, et al. Diagnostic value of novel biomarkers for heart failure : A meta-analysis. Herz 2020;45:65-78. [Crossref] [PubMed]
- McCarthy CP, Januzzi JL Jr. Soluble ST2 in Heart Failure. Heart Fail Clin 2018;14:41-8. [Crossref] [PubMed]
- Pascual-Figal DA, Ordoñez-Llanos J, Tornel PL, et al. Soluble ST2 for predicting sudden cardiac death in patients with chronic heart failure and left ventricular systolic dysfunction. J Am Coll Cardiol 2009;54:2174-9. [Crossref] [PubMed]
- Manzano-Fernández S, Mueller T, Pascual-Figal D, et al. Usefulness of soluble concentrations of interleukin family member ST2 as predictor of mortality in patients with acutely decompensated heart failure relative to left ventricular ejection fraction. Am J Cardiol 2011;107:259-67. [Crossref] [PubMed]
- Stenemo M, Nowak C, Byberg L, et al. Circulating proteins as predictors of incident heart failure in the elderly. Eur J Heart Fail 2018;20:55-62. [Crossref] [PubMed]
- Savvoulidis P, Snider JV, Rawal S, et al. Serum ST2 and hospitalization rates in Caucasian and African American outpatients with heart failure. Int J Cardiol 2020;304:116-21. [Crossref] [PubMed]
- Najjar E, Faxén UL, Hage C, et al. ST2 in heart failure with preserved and reduced ejection fraction. Scand Cardiovasc J 2019;53:21-7. [Crossref] [PubMed]
- Tsigkou V, Siasos G, Bletsa E, et al. The Predictive Role for ST2 in Patients with Acute Coronary Syndromes and Heart Failure. Curr Med Chem 2020;27:4479-93. [Crossref] [PubMed]
- Bayés-Genís A, Núñez J, Lupón J. Soluble ST2 for Prognosis and Monitoring in Heart Failure: The New Gold Standard? J Am Coll Cardiol 2017;70:2389-92. [Crossref] [PubMed]
- Revuelta-López E, Lupón J, Lax A, et al. Differences in the Interleukin-1β/Soluble ST2 Interplay Between Acute and Chronic Heart Failure. J Cardiovasc Transl Res 2020;13:864-6. [Crossref] [PubMed]
- Berezin AE. Prognostication of clinical outcomes in diabetes mellitus: Emerging role of cardiac biomarkers. Diabetes Metab Syndr 2019;13:995-1003. [Crossref] [PubMed]
- Zhong X, Qian X, Chen G, et al. The role of galectin-3 in heart failure and cardiovascular disease. Clin Exp Pharmacol Physiol 2019;46:197-203. [Crossref] [PubMed]
- Suthahar N, Meijers WC, Silljé HHW, et al. Galectin-3 Activation and Inhibition in Heart Failure and Cardiovascular Disease: An Update. Theranostics 2018;8:593-609. [Crossref] [PubMed]
- Song X, Qian X, Shen M, et al. Protein kinase C promotes cardiac fibrosis and heart failure by modulating galectin-3 expression. Biochim Biophys Acta 2015;1853:513-21. [Crossref] [PubMed]
- Mishra S, Kass DA. Cellular and molecular pathobiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2021;18:400-23. [Crossref] [PubMed]
- de Boer RA, Edelmann F, Cohen-Solal A, et al. Galectin-3 in heart failure with preserved ejection fraction. Eur J Heart Fail 2013;15:1095-101. [Crossref] [PubMed]
- Yu X, Sun Y, Zhao Y, et al. Prognostic value of plasma galectin-3 levels in patients with coronary heart disease and chronic heart failure. Int Heart J 2015;56:314-8. [Crossref] [PubMed]
- Trippel TD, Mende M, Düngen HD, et al. The diagnostic and prognostic value of galectin-3 in patients at risk for heart failure with preserved ejection fraction: results from the DIAST-CHF study. ESC Heart Fail 2021;8:829-41. [Crossref] [PubMed]
- de Boer RA, Lok DJ, Jaarsma T, et al. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann Med 2011;43:60-8. [Crossref] [PubMed]
- de Boer RA, Nayor M, deFilippi CR, et al. Association of Cardiovascular Biomarkers With Incident Heart Failure With Preserved and Reduced Ejection Fraction. JAMA Cardiol 2018;3:215-24. [Crossref] [PubMed]
- Kanukurti J, Mohammed N, Sreedevi NN, et al. Evaluation of Galectin-3 as a Novel Diagnostic Biomarker in Patients with Heart Failure with Preserved Ejection Fraction. J Lab Physicians 2020;12:126-32. [Crossref] [PubMed]
- Vora A, de Lemos JA, Ayers C, et al. Association of Galectin-3 With Diabetes Mellitus in the Dallas Heart Study. J Clin Endocrinol Metab 2019;104:4449-58. [Crossref] [PubMed]
- Nayor M, Wang N, Larson MG, et al. Circulating Galectin-3 Is Associated With Cardiometabolic Disease in the Community. J Am Heart Assoc 2015;5:002347. [PubMed]
- Biasucci LM, Maino A, Grimaldi MC, et al. Novel Biomarkers in Heart Failure: New Insight in Pathophysiology and Clinical Perspective. J Clin Med 2021;10:2771. [Crossref] [PubMed]
- Demaison L. Oxidative Stress and Obesity- and Type 2 Diabetes-Induced Heart Failure. Antioxidants (Basel) 2020;9:653. [Crossref] [PubMed]
- Aimo A, Castiglione V, Borrelli C, et al. Oxidative stress and inflammation in the evolution of heart failure: From pathophysiology to therapeutic strategies. Eur J Prev Cardiol 2020;27:494-510. [Crossref] [PubMed]
- Milinković I, Polovina M, Simeunović DS, et al. Oxidative stress and inflammation in heart failure: The best is yet to come. Eur J Prev Cardiol 2020;27:490-3. [Crossref] [PubMed]
- Kotlyar E, Vita JA, Winter MR, et al. The relationship between aldosterone, oxidative stress, and inflammation in chronic, stable human heart failure. J Card Fail 2006;12:122-7. [Crossref] [PubMed]
- Kohen Avramoglu R, Laplante MA, Le Quang K, et al. The genetic and metabolic determinants of cardiovascular complications in type 2 diabetes: recent insights from animal models and clinical investigations. Can J Diabetes 2013;37:351-8. [Crossref] [PubMed]
- Zhu Y, Pan W, Yang T, et al. Upregulation of Circular RNA CircNFIB Attenuates Cardiac Fibrosis by Sponging miR-433. Front Genet 2019;10:564. [Crossref] [PubMed]
- Nagpal V, Rai R, Place AT, et al. MiR-125b Is Critical for Fibroblast-to-Myofibroblast Transition and Cardiac Fibrosis. Circulation 2016;133:291-301. [Crossref] [PubMed]
- Wang J, Zhang S, Li X, et al. LncRNA SNHG7 promotes cardiac remodeling by upregulating ROCK1 via sponging miR-34-5p. Aging (Albany NY) 2020;12:10441-56. [Crossref] [PubMed]
- Gurkar AU, Chu K, Raj L, et al. Identification of ROCK1 kinase as a critical regulator of Beclin1-mediated autophagy during metabolic stress. Nat Commun 2013;4:2189. [Crossref] [PubMed]
- Patel VB, Mori J, McLean BA, et al. ACE2 Deficiency Worsens Epicardial Adipose Tissue Inflammation and Cardiac Dysfunction in Response to Diet-Induced Obesity. Diabetes 2016;65:85-95. [Crossref] [PubMed]
- Matloch Z, Cinkajzlova A, Mraz M, et al. The Role of Inflammation in Epicardial Adipose Tissue in Heart Diseases. Curr Pharm Des 2018;24:297-309. [Crossref] [PubMed]
- Park HK, Kwak MK, Kim HJ, et al. Linking resistin, inflammation, and cardiometabolic diseases. Korean J Intern Med 2017;32:239-47. [Crossref] [PubMed]
- Valero-Muñoz M, Li S, Wilson RM, et al. Heart Failure With Preserved Ejection Fraction Induces Beiging in Adipose Tissue. Circ Heart Fail 2016;9:e002724. [Crossref] [PubMed]
- Masson S, Gori F, Latini R, et al. Adiponectin in chronic heart failure: influence of diabetes and genetic variants. Eur J Clin Invest 2011;41:1330-8. [Crossref] [PubMed]
- Frankel DS, Vasan RS, D'Agostino RB Sr, et al. Resistin, adiponectin, and risk of heart failure the Framingham offspring study. J Am Coll Cardiol 2009;53:754-62. [Crossref] [PubMed]
- Gualillo O, González-Juanatey JR, Lago F. The emerging role of adipokines as mediators of cardiovascular function: physiologic and clinical perspectives. Trends Cardiovasc Med 2007;17:275-83. [Crossref] [PubMed]
- Schram K, Sweeney G. Implications of myocardial matrix remodeling by adipokines in obesity-related heart failure. Trends Cardiovasc Med 2008;18:199-205. [Crossref] [PubMed]
- Azizi Ghanbari A, Dörr R, Spitzer S, et al. Adiponectin in coronary heart disease and newly diagnosed impaired glucose tolerance. Diab Vasc Dis Res 2013;10:452-8. [Crossref] [PubMed]
- Djoussé L, Wilk JB, Hanson NQ, et al. Association between adiponectin and heart failure risk in the physicians' health study. Obesity (Silver Spring) 2013;21:831-4. [Crossref] [PubMed]
- Menzel J, di Giuseppe R, Biemann R, et al. Association between chemerin, omentin-1 and risk of heart failure in the population-based EPIC-Potsdam study. Sci Rep 2017;7:14171. [Crossref] [PubMed]
- Sans-Roselló J, Casals G, Rossello X, et al. Prognostic value of plasma apelin concentrations at admission in patients with ST-segment elevation acute myocardial infarction. Clin Biochem 2017;50:279-84. [Crossref] [PubMed]
- Zhou X, Tao Y, Chen Y, et al. Serum Chemerin as a Novel Prognostic Indicator in Chronic Heart Failure. J Am Heart Assoc 2019;8:e012091. [Crossref] [PubMed]
- Brankovic M, Akkerhuis KM, Mouthaan H, et al. Cardiometabolic Biomarkers and Their Temporal Patterns Predict Poor Outcome in Chronic Heart Failure (Bio-SHiFT Study). J Clin Endocrinol Metab 2018;103:3954-64. [Crossref] [PubMed]
- Zheng M, Lu N, Ren M, et al. Visfatin associated with major adverse cardiovascular events in patients with acute myocardial infarction. BMC Cardiovasc Disord 2020;20:271. [Crossref] [PubMed]
- Ahmed HH, Shousha WG, El-Mezayen HA, et al. New Biomarkers as Prognostic Factors for Cardiovascular Complications in Type 2 Diabetic Patients. Indian J Clin Biochem 2020;35:54-62. [Crossref] [PubMed]
- Xu CC, Fu GX, Liu QQ, et al. Association between cystatin C and heart failure with preserved ejection fraction in elderly Chinese patients. Z Gerontol Geriatr 2018;51:92-7. [Crossref] [PubMed]
- Jirak P, Stechemesser L, Moré E, et al. Clinical implications of fetuin-A. Adv Clin Chem 2019;89:79-130. [Crossref] [PubMed]
- Lichtenauer M, Wernly B, Paar V, et al. Specifics of fetuin-A levels in distinct types of chronic heart failure. J Clin Lab Anal 2018; [Crossref] [PubMed]
- Arnaud P, Kalabay L. Alpha2-HS glycoprotein: a protein in search of a function. Diabetes Metab Res Rev 2002;18:311-4. [Crossref] [PubMed]
- Srinivas PR, Wagner AS, Reddy LV, et al. Serum alpha 2-HS-glycoprotein is an inhibitor of the human insulin receptor at the tyrosine kinase level. Mol Endocrinol 1993;7:1445-55. [PubMed]
- Stefan N, Hennige AM, Staiger H, et al. Alpha2-Heremans-Schmid glycoprotein/fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care 2006;29:853-7. [Crossref] [PubMed]
- Mori K, Emoto M, Yokoyama H, et al. Association of serum fetuin-A with insulin resistance in type 2 diabetic and nondiabetic subjects. Diabetes Care 2006;29:468. [Crossref] [PubMed]
- Sun Q, Cornelis MC, Manson JE, et al. Plasma levels of fetuin-A and hepatic enzymes and risk of type 2 diabetes in women in the U.S. Diabetes 2013;62:49-55. [Crossref] [PubMed]
- Stefan N, Fritsche A, Weikert C, et al. Plasma fetuin-A levels and the risk of type 2 diabetes. Diabetes 2008;57:2762-7. [Crossref] [PubMed]
- Ix JH, Biggs ML, Mukamal KJ, et al. Association of fetuin-a with incident diabetes mellitus in community-living older adults: the cardiovascular health study. Circulation 2012;125:2316-22. [Crossref] [PubMed]
- Guo VY, Cao B, Cai C, et al. Fetuin-A levels and risk of type 2 diabetes mellitus: a systematic review and meta-analysis. Acta Diabetol 2018;55:87-98. [Crossref] [PubMed]
- Sujana C, Huth C, Zierer A, et al. Association of fetuin-A with incident type 2 diabetes: results from the MONICA/KORA Augsburg study and a systematic meta-analysis. Eur J Endocrinol 2018;178:389-98. [Crossref] [PubMed]
- Roshanzamir F, Miraghajani M, Rouhani MH, et al. The association between circulating fetuin-A levels and type 2 diabetes mellitus risk: systematic review and meta-analysis of observational studies. J Endocrinol Invest 2018;41:33-47. [Crossref] [PubMed]
- Icer MA, Yıldıran H. Effects of fetuin-A with diverse functions and multiple mechanisms on human health. Clin Biochem 2021;88:1-10. [Crossref] [PubMed]
- Mathews ST, Rakhade S, Zhou X, et al. Fetuin-null mice are protected against obesity and insulin resistance associated with aging. Biochem Biophys Res Commun 2006;350:437-43. [Crossref] [PubMed]
- Hennige AM, Staiger H, Wicke C, et al. Fetuin-A induces cytokine expression and suppresses adiponectin production. PLoS One 2008;3:e1765. [Crossref] [PubMed]
- Alford AI, Hankenson KD. Matricellular proteins: Extracellular modulators of bone development, remodeling, and regeneration. Bone 2006;38:749-57. [Crossref] [PubMed]
- Schellings MW, Pinto YM, Heymans S. Matricellular proteins in the heart: possible role during stress and remodeling. Cardiovasc Res 2004;64:24-31. [Crossref] [PubMed]
- Berezin AE. Bone-Related Proteins as Markers in Vascular Remodeling. In: Preedy V. editor. Biomarkers in Bone Disease. Dordrecht: Springer, 2015:1-22.
- Frangogiannis NG, Kovacic JC. Extracellular Matrix in Ischemic Heart Disease, Part 4/4: JACC Focus Seminar. J Am Coll Cardiol 2020;75:2219-35. [Crossref] [PubMed]
- Behnes M, Brueckmann M, Lang S, et al. Diagnostic and prognostic value of osteopontin in patients with acute congestive heart failure. Eur J Heart Fail 2013;15:1390-400. [Crossref] [PubMed]
- Schellings MW, Vanhoutte D, Swinnen M, et al. Absence of SPARC results in increased cardiac rupture and dysfunction after acute myocardial infarction. J Exp Med 2009;206:113-23. [Crossref] [PubMed]
- Berezin AE, Kremzer AA. Predictive value of circulating osteonectin in patients with ischemic symptomatic chronic heart failure. Biomed J 2015;38:523-30. [Crossref] [PubMed]
- Timotin A, Cinato M, Boal F, et al. Differential protein profiling as a potential multi-marker approach for obese patients with heart failure: A retrospective study. Sci Rep 2018;8:7894. [Crossref] [PubMed]
- Adela R, Banerjee SK. GDF-15 as a Target and Biomarker for Diabetes and Cardiovascular Diseases: A Translational Prospective. J Diabetes Res 2015;2015:490842. [Crossref] [PubMed]
- Tsai VWW, Husaini Y, Sainsbury A, et al. The MIC-1/GDF15-GFRAL Pathway in Energy Homeostasis: Implications for Obesity, Cachexia, and Other Associated Diseases. Cell Metab 2018;28:353-68. [Crossref] [PubMed]
- Breit SN, Brown DA, Tsai VW. The GDF15-GFRAL Pathway in Health and Metabolic Disease: Friend or Foe? Annu Rev Physiol 2021;83:127-51. [Crossref] [PubMed]
- Emmerson PJ, Wang F, Du Y, et al. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat Med 2017;23:1215-9. [Crossref] [PubMed]
- Kempf T, Guba-Quint A, Torgerson J, et al. Growth differentiation factor 15 predicts future insulin resistance and impaired glucose control in obese nondiabetic individuals: results from the XENDOS trial. Eur J Endocrinol 2012;167:671-8. [Crossref] [PubMed]
- Echouffo-Tcheugui JB, Daya N, Matsushita K, et al. Growth Differentiation Factor (GDF)-15 and Cardiometabolic Outcomes among Older Adults: The Atherosclerosis Risk in Communities Study. Clin Chem 2021;67:653-61. [Crossref] [PubMed]
- Sanders-van Wijk S, Tromp J, Beussink-Nelson L, et al. Proteomic Evaluation of the Comorbidity-Inflammation Paradigm in Heart Failure With Preserved Ejection Fraction: Results From the PROMIS-HFpEF Study. Circulation 2020;142:2029-44. [Crossref] [PubMed]
- Kanagala P, Arnold JR, Singh A, et al. Characterizing heart failure with preserved and reduced ejection fraction: An imaging and plasma biomarker approach. PLoS One 2020;15:e0232280. [Crossref] [PubMed]
- Kempf T, von Haehling S, Peter T, et al. Prognostic utility of growth differentiation factor-15 in patients with chronic heart failure. J Am Coll Cardiol 2007;50:1054-60. [Crossref] [PubMed]
- Tuegel C, Katz R, Alam M, et al. GDF-15, Galectin 3, Soluble ST2, and Risk of Mortality and Cardiovascular Events in CKD. Am J Kidney Dis 2018;72:519-28. [Crossref] [PubMed]
- Rabkin SW, Tang JKK. The utility of growth differentiation factor-15, galectin-3, and sST2 as biomarkers for the diagnosis of heart failure with preserved ejection fraction and compared to heart failure with reduced ejection fraction: a systematic review. Heart Fail Rev 2021;26:799-812. [Crossref] [PubMed]
- Bouabdallaoui N, Claggett B, Zile MR, et al. Growth differentiation factor-15 is not modified by sacubitril/valsartan and is an independent marker of risk in patients with heart failure and reduced ejection fraction: the PARADIGM-HF trial. Eur J Heart Fail 2018;20:1701-9. [Crossref] [PubMed]
- Breidthardt T, Sabti Z, Ziller R, et al. Diagnostic and prognostic value of cystatin C in acute heart failure. Clin Biochem 2017;50:1007-13. [Crossref] [PubMed]
- Chen S, Tang Y, Zhou X. Cystatin C for predicting all-cause mortality and rehospitalization in patients with heart failure: a meta-analysis. Biosci Rep 2019;39:BSR20181761. [Crossref] [PubMed]
- Huerta A, López B, Ravassa S, et al. Association of cystatin C with heart failure with preserved ejection fraction in elderly hypertensive patients: potential role of altered collagen metabolism. J Hypertens 2016;34:130-8. [Crossref] [PubMed]
- van der Laan SW, Fall T, Soumaré A, et al. Cystatin C and Cardiovascular Disease: A Mendelian Randomization Study. J Am Coll Cardiol 2016;68:934-45. [Crossref] [PubMed]
- Palazzuoli A, Beltrami M, Pellegrini M, et al. Natriuretic peptides and NGAL in heart failure: does a link exist? Clin Chim Acta 2012;413:1832-8. [Crossref] [PubMed]
- Oikonomou E, Tsalamandris S, Karlis D, et al. The association among biomarkers of renal and heart function in patients with heart failure: the role of NGAL. Biomark Med 2018;12:1323-30. [Crossref] [PubMed]
- Marques FZ, Prestes PR, Byars SG, et al. Experimental and Human Evidence for Lipocalin-2 (Neutrophil Gelatinase-Associated Lipocalin [NGAL]) in the Development of Cardiac Hypertrophy and heart failure. J Am Heart Assoc 2017;6:e005971. [Crossref] [PubMed]
- Lábr K, Špinar J, Pařenica J, et al. Renal Functions and Prognosis Stratification in Chronic Heart Failure Patients and the Importance of Neutrophil Gelatinase-Associated Lipocalin. Kidney Blood Press Res 2018;43:1865-77. [Crossref] [PubMed]
- Maisel AS, Mueller C, Fitzgerald R, et al. Prognostic utility of plasma neutrophil gelatinase-associated lipocalin in patients with acute heart failure: the NGAL EvaLuation Along with B-type NaTriuretic Peptide in acutely decompensated heart failure (GALLANT) trial. Eur J Heart Fail 2011;13:846-51. [Crossref] [PubMed]
- Mukherji A, Ansari U, Borggrefe M, et al. Clinically Relevant Biomarkers in Acute Heart Failure: An Update. Curr Pharm Biotechnol 2017;18:482-90. [Crossref] [PubMed]
- Bielecka-Dabrowa A, Gluba-Brzózka A, Michalska-Kasiczak M, et al. The multi-biomarker approach for heart failure in patients with hypertension. Int J Mol Sci 2015;16:10715-33. [Crossref] [PubMed]
- van Deursen VM, Damman K, Voors AA, et al. Prognostic value of plasma neutrophil gelatinase-associated lipocalin for mortality in patients with heart failure. Circ Heart Fail 2014;7:35-42. [Crossref] [PubMed]
- Emmens JE, Ter Maaten JM, Matsue Y, et al. Plasma kidney injury molecule-1 in heart failure: renal mechanisms and clinical outcome. Eur J Heart Fail 2016;18:641-9. [Crossref] [PubMed]
- Grodin JL, Perez AL, Wu Y, et al. Circulating Kidney Injury Molecule-1 Levels in Acute Heart Failure: Insights From the ASCEND-HF Trial (Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure). JACC Heart Fail 2015;3:777-85. [Crossref] [PubMed]
- Atici A, Emet S, Cakmak R, et al. Type I cardiorenal syndrome in patients with acutely decompensated heart failure: the importance of new renal biomarkers. Eur Rev Med Pharmacol Sci 2018;22:3534-43. [PubMed]
- Berezin AE, Berezin AA, Lichtenauer M. Myokines and Heart Failure: Challenging Role in Adverse Cardiac Remodeling, Myopathy, and Clinical Outcomes. Dis Markers 2021;2021:6644631. [Crossref] [PubMed]
- Maltais S, Perrault LP, Ly HQ. The bone marrow-cardiac axis: role of endothelial progenitor cells in heart failure. Eur J Cardiothorac Surg 2011;39:368-74. [Crossref] [PubMed]
- Samman Tahhan A, Hammadah M, Sandesara PB, et al. Progenitor Cells and Clinical Outcomes in Patients With Heart Failure. Circ Heart Fail 2017;10:e004106. [Crossref] [PubMed]
- Berezin AE, Kremzer AA, Martovitskaya YV, et al. Pattern of endothelial progenitor cells and apoptotic endothelial cell-derived microparticles in chronic heart failure patients with preserved and reduced left ventricular ejection fraction. EBioMedicine 2016;4:86-94. [Crossref] [PubMed]
- Koller L, Hohensinner P, Sulzgruber P, et al. Prognostic relevance of circulating endothelial progenitor cells in patients with chronic heart failure. Thromb Haemost 2016;116:309-16. [Crossref] [PubMed]
- Magkoutis N, Mantzaraki V, Farmakis D, et al. Effects of functional electrical stimulation of lower limb muscles on circulating endothelial progenitor cells, CD34+ cells and vascular endothelial growth factor-A in heart failure with reduced ejection fraction. Eur J Heart Fail 2018;20:1162-3. [Crossref] [PubMed]
- Berezin AE, Kremzer AA. Circulating endothelial progenitor cells as markers for severity of ischemic chronic heart failure. J Card Fail 2014;20:438-47. [Crossref] [PubMed]
- Premer C, Blum A, Bellio MA, et al. Allogeneic Mesenchymal Stem Cells Restore Endothelial Function in Heart Failure by Stimulating Endothelial Progenitor Cells. EBioMedicine 2015;2:467-75. [Crossref] [PubMed]
- Müller P, Beltrami AP, Cesselli D, et al. Myocardial regeneration by endogenous adult progenitor cells. J Mol Cell Cardiol 2005;39:377-87. [Crossref] [PubMed]
- Khan S, Rasool ST. Current Use of Cardiac Biomarkers in Various Heart Conditions. Endocr Metab Immune Disord Drug Targets 2021;21:980-93. [Crossref] [PubMed]
- Düngen HD, Tscholl V, Obradovic D, et al. Prognostic performance of serial in-hospital measurements of copeptin and multiple novel biomarkers among patients with worsening heart failure: results from the MOLITOR study. ESC Heart Fail 2018;5:288-96. [Crossref] [PubMed]
- Zhang M, Meng Q, Qi X, et al. Comparison of multiple biomarkers for mortality prediction in patients with acute heart failure of ischemic and nonischemic etiology. Biomark Med 2018;12:1207-17. [Crossref] [PubMed]
- Pandey A, Vaduganathan M, Patel KV, et al. Biomarker-Based Risk Prediction of Incident Heart Failure in Pre-Diabetes and Diabetes. JACC Heart Fail 2021;9:215-23. [Crossref] [PubMed]
- Berezin AE, Kremzer AA, Samura TA, et al. Altered signature of apoptotic endothelial cell-derived microvesicles predicts chronic heart failure phenotypes. Biomark Med 2019;13:737-50. [Crossref] [PubMed]
- Berezin AE, Kremzer AA, Martovitskaya YV, et al. The utility of biomarker risk prediction score in patients with chronic heart failure. Int J Clin Exp Med 2015;8:18255-64. [Crossref] [PubMed]
- Schernthaner C, Lichtenauer M, Wernly B, et al. Multibiomarker analysis in patients with acute myocardial infarction. Eur J Clin Invest 2017;47:638-48. [Crossref] [PubMed]
- Huttin O, Kobayashi M, Ferreira JP, et al. Circulating multimarker approach to identify patients with preclinical left ventricular remodelling and/or diastolic dysfunction. ESC Heart Fail 2021;8:1700-5. [Crossref] [PubMed]
Cite this article as: Berezin AE, Lichtenauer M, Berezin AA. Heart failure among patients with prediabetes and type 2 diabetes mellitus: diagnostic and predictive biomarkers: a narrative review. J Lab Precis Med 2022;7:5.


