Accuracy of procalcitonin in identifying patients presenting with diabetic ketoacidosis as a result of an infectious etiology
Introduction
Diabetic ketoacidosis (DKA) is a life-threatening condition, accounting for 4–9% of all hospital discharges among diabetic patients (1). Common presentations of DKA include new-onset diabetes, discontinuation or non-compliance with medications, and significant bodily stress which includes (but not limited to) trauma, heart attacks, strokes, pancreatitis, and infections. The mainstay treatment of DKA includes aggressive fluid resuscitation, replacing insulin stores, and correction of electrolyte disturbances. Additionally, providers must identify and treat the precipitating cause of DKA (2).
Early detection and treatment of infections are key elements to improved patient outcomes, and as such, empiric antibiotics are frequently prescribed to patients who present with DKA. Patient presentation characteristics are quite similar in DKA as well as infections, thus resulting in excessive antibiotic use. This can lead to increased treatment costs, medication side effects, and the risk of development of antibiotic resistance. Over the last decade, the development and usage of clinical biomarkers [such as C-reactive protein (CRP) and procalcitonin (PCT)] have greatly assisted clinicians in differentiating patient presentations related to infections compared to other conditions (3). This has led to optimized and appropriate antibiotic usage while improving resource utilization as well as hospital costs (4).
PCT is a 116-amino acid protein precursor to calcitonin produced by neuroendocrine cells, including thyroid C cells. PCT serves as one of the major biomarkers for the diagnosis of bacterial infections. In healthy patients, it is not produced in significant amounts; however, in the presence of severe systemic inflammation because of bacterial infection, measurable quantities of PCT can be produced. Several randomized controlled trials have found that measurement of PCT can be used to successfully shorten the duration of antimicrobial therapy in sepsis and lower respiratory tract infections (1,5). PCT measurement can also serve to avoid antibiotic initiation altogether (6).
To our knowledge, there have been no prospective evaluations of PCT levels in patients presenting with DKA. The objective of this study is to assess the utility and trend of PCT in DKA. If PCT proves to be an effective biomarker in identifying infection in patients with DKA, this can prevent unnecessary use of antibiotics or shorten the duration of antibiotic use in patients who have a true infection. We present the following article in accordance with the STARD reporting checklist (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-21-38/rc).
Methods
Design and patients
This was a prospective, observational study performed at a single, community-academic medical center and included confirmed consecutive patients with DKA admitted to the intensive care unit from August 2018 to May 2020. Patients were eligible for the study if they met the following criteria: blood glucose over 250 mg/dL, evidence of ketonuria, either blood pH of less than 7.30 or anion gap calculated to be greater than 14, and capacity to give informed consent. Patients were excluded if they were under 18 years of age, routine blood draws were not obtained as a part of the patients’ routine care during time intervals specified by the protocol, patient was unable to consent due to clinical condition, or if the available IV access failed to draw back blood. The primary PCT blood sample was obtained upon patient consent within the first 24 hours of admission and DKA diagnosis and a second was drawn within 48–72 hours if the first PCT level was above the threshold for suspected infection (≥0.25 ng/mL). All PCT levels were blinded from treating physicians as to not impact clinical decision making, unless ordered on the admitting physicians own accord.
Ethics
The trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Saint Barnabas Medical Center (IRB protocol 18-29) and informed consent was taken from all individual participants. No additional venipuncture occurred as a result of this study and was only obtained from an intravenous access point with blood drawback or during routine blood work. Any additional diagnostic testing and treatment of DKA occurred by the admitting clinician independent of study participation and investigators.
End points and statistical analysis
The primary endpoint evaluated the percentage of patients with DKA with microbiologically or clinically confirmed bacterial infection and PCT ≥0.25 ng/mL. If microbiology results were not collected by the primary care team, the participant was presumed not to have an infection. Patients were classified as having proven bacterial infection (PBI) based the presence of positive microbiologic cultures, new pulmonary infiltrate on chest radiograph, and symptoms of infection. Secondary outcomes reviewed specificity and negative predictive value of the test based on microbiologic or clinical diagnosis compared with a PCT value of <0.25 ng/mL. Additional secondary outcomes included antibiotic days and intensive care unit length of stay. The trends of PCT and continued clinical/microbiological evidence for persistent infection was assessed at 48 to 72 hours with a follow up PCT level. The intended sample size was set at 100 participants due to the paucity of data. The study terminated early after the inclusion of 86 patients due to the onset of the COVID-19 virus in March of 2020. The study investigators were concerned about the unknown effect of this virus on the treatment of DKA.
The association between patient characteristics and infection were assessed using the Wilcoxon rank-sum test for continuous characteristics and the Chi-square or Fisher’s exact test for categorical characteristics. Spearman rank correlation was used to assess the correlation between PCT and creatinine clearance. Sensitivity, specificity, positive predictive values, negative predictive values, receiver operating characteristic curves (ROC), and areas under the curves (AUC) were calculated. All analyses were completed in R 4.0.2.
Results
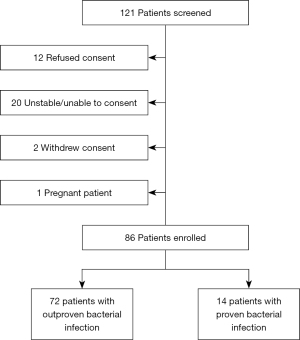
A total of 121 patients with confirmed DKA were screened to participate in the study. The final analysis included 86 patients who consented to participate. Proven bacterial infection (PBI) was confirmed in 14 (16.3%) patients with sources of infection delineated in Figure 1. Baseline characteristics (Table 1) that varied most between the PBI and non-PBI groups include higher patient median age, female sex, and presence of positive SIRS criteria (2 out of 4) among patients with PBI. Although the difference was not statistically significant, the median creatinine clearance on admission was lower among patients with PBI vs. non-PBI patients (36.6 vs. 54.8 mL/min, P=0.089). Admission blood sugar, lactate, and HgA1c were similar between both groups.
Table 1
| Variable | Proven bacterial infection | P value | |
|---|---|---|---|
| No (n=72) | Yes (n=14) | ||
| Age, years | 45.5 (19.0, 89.0) | 64.0 (22.0, 85.0) | 0.005 |
| Sex | 0.011 | ||
| Female | 32 (44.4) | 12 (85.7) | |
| Male | 40 (55.6) | 2 (14.3) | |
| Blood sugar (mmol/L) | 537 (142, 1,481) | 569 (369, 1,175) | 0.098 |
| HbA1c | 11.8 (6.2, 18.5) | 11.8 (7.5, 14.4) | 0.927 |
| Lactate | 2.4 (0.9, 11.9) | 3.7 (1.7, 4.9) | 0.214 |
| ABG pH | 7.2 (0.1, 35.0) | 7.3 (6.8, 7.4) | 0.258 |
| Temperature (°F) | 98.1 (92.3, 100.4) | 98.3 (90.6, 100.0) | 0.512 |
| Heart rate (bpm) | 106 (61, 154) | 108 (89, 136) | 0.215 |
| Respiratory rate (per minute) | 20 (16, 30) | 22 (16, 26) | 0.627 |
| White blood cell count (×109/L) | 11.7 (3.1, 26.3) | 15.6 (4.2, 28.8) | 0.045 |
| SIRS criteria | 0.024 | ||
| Negative | 37 (51.4) | 2 (14.3) | |
| Positive | 35 (48.6) | 12 (85.7) | |
| Creatinine clearance - admit | 54.8 (2.4, 163.0) | 36.6 (17.2, 119.4) | 0.089 |
| <60 mL/min | 40 (55.6) | 10 (71.4) | 0.421 |
| >60 mL/min | 32 (44.4) | 4 (28.6) | |
Data are presented as median (range) or n (%). ABG, arterial blood gas; SIRS, systemic inflammatory response syndrome.
The primary outcome evaluating the median PCT at admission did not differ significantly among non-PBI patients vs. PBI patients (0.3 vs. 0.5 ng/mL; P=0.068) (Table 2). A greater proportion of patients in the non-PBI group (45.8%) compared to PBI group (21.4%) had PCT levels <0.25 ng/mL (Figure 2). Obtaining follow-up laboratory PCT levels at 48 to 72 hours was not completed in all patients due to transfer from the ICU or hospital discharge. Seventeen patients had a PCT repeated within the 72-hour time window, which demonstrated a trend towards higher PCT levels in the PBI group vs. non-PBI group (1.28 vs. 0.27 ng/mL). There were no unanticipated adverse events for patients as prescribers were blinded to study testing.
Table 2
| Variable | Proven bacterial infection | P value | |
|---|---|---|---|
| No (n=72) | Yes (n=14) | ||
| Admission procalcitonin (ng/mL) | 0.3 (0.0, 19.2) | 0.5 (0.1, 174.2) | 0.068 |
| Admission procalcitonin group | 0.162 | ||
| <0.25 ng/mL | 33 (45.8) | 3 (21.4) | |
| ≥0.25 ng/mL | 39 (54.2) | 11 (78.6) | |
| ICU length of stay, days | 2 (1, 6) | 3 (1, 5) | 0.011 |
| Median procalcitonin 48 to 72 hours (ng/mL)* | 0.267 | 1.284 | – |
Data are presented as median (range) or n (%). *, no (n=11), yes (n=14).
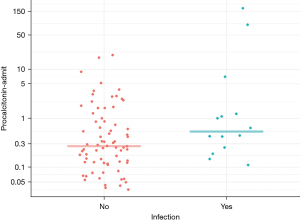
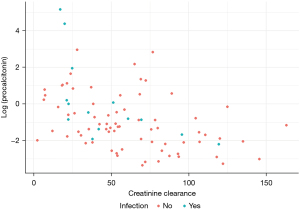
Furthermore, in a post hoc analysis, among the subset with creatinine clearance (CrCl) of >30 mL/min, no statistically significant difference was observed in PCT values amongst the PBI and non-PBI groups (median 0.2 vs. 0.3, P=0.411). Additionally, CrCl was found to have a negative correlation with PCT (spearman rank correlation =−0.49) (Figure 3).
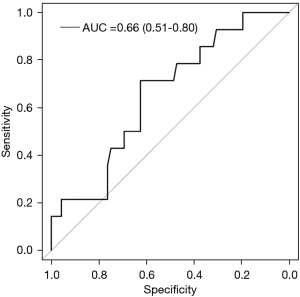
Secondary outcomes evaluating the average duration of empiric antibiotic exposure among the subset of patients without PBI (n=20) vs. patients with PBI (n=13) was 0.94 vs. 11.14 days. Intensive care unit length of stay (LOS) was significantly shorter in patients with PBI than with those without PBI (median 3 vs. 2 days, P=0.011). Using the threshold PCT level for suspected infection (≥0.25 ng/mL) the positive predictive value for bacterial infection was calculated to be 22%. Conversely, the negative predictive value was 91.6%. Sensitivity for PCT in DKA was 78% (95% CI: 0.57–1.00) and specificity was 45.8% (95% CI: 0.34–0.57). Figure 4 is the receiver operator characteristic curve for PCT in DKA patients AUC =0.66 (95% CI: 0.51–0.80).
Discussion
Our study is the first prospective assessment of PCT demonstrating the reliability of identifying infection in patients admitted with DKA. We demonstrate that there is a trend towards a higher PCT level in patients with infection in the setting of DKA. Our data suggests the negative predictive value of PCT is strong and antibiotic treatment should be deferred if a patient with DKA has a negative PCT on admission.
There are several limitations to our prospective study. First, this is a single-center trial with a limited patient population which limits the power of the study. Secondly, there were challenges in capturing day 2 follow up PCT levels due to transfer within the healthcare facility and/or patient discharge leading to a loss in follow up. Additionally, we selected the lower respiratory tract value for PCT 0.25 ng/mL to guide our analysis as opposed to the 0.5 ng/mL value for sepsis because antibiotics would be indicated for lower respiratory tract infection despite the presumption of a less severe infection. Lastly, the number of confirmed infections was low as the large majority of DKA cases were due to new-onset diabetes, noncompliance, and/or other non-infectious causes.
Despite these limitations, the results of our study demonstrate the potential utility of PCT in avoiding antimicrobial therapy for patients who present with DKA without overt bacterial infection or sepsis. Our findings are similar to that of previous retrospective studies evaluating PCT. Ivaska et al. evaluated the correlation of C-reactive protein and PCT with the severity of illness in a retrospective study of acutely ill pediatric patients. This study included four teenage patients with DKA and demonstrated that with worsening acidosis and hyperglycemia there was a greater elevation in PCT. While none of these patients had overt signs of bacterial infection, two of the four patients received antibiotic therapy. The range of PCT values identified included 0.05 to 82.94 mcg/L (6). Another retrospective chart review study of adult patients with DKA in France sought to establish the role of PCT similar to our study. They reviewed cases for proven bacterial infection (PBI) based on the presence of positive bacterial cultures in 102 episodes of DKA and 20 cases were classified as PBI. All of the cases with PBI were treated with antibiotic therapy, while in cases without PBI 31% received empiric antibiotic therapy. Temperature, fever rate, PCT were found to be significantly elevated in PBI cases compared to non-PBI cases on univariate analysis. A multivariate analysis adjusted age, insulin treatment, ketones, the severity of illness score was also conducted. The elevated PCT above 1.44 ng/mL (OR 1.27 95% CI: 1.04–1.63) and fever (OR 27.86, 95% CI: 1.97–887.92) were associated with PBI. In the patients, without PBI the mean PCT was 0.52 and 0.42 ng/mL on hospital admission and day 2 respectively (7). Both of these retrospective studies and our prospective observational data confirm that if PCT value is not elevated in DKA, clinicians may consider treatment of DKA without concern for bacterial infection or initiation of antibiotic therapy.
The mechanism of elevation of PCT of DKA is unclear. There are several conditions known to contribute to elevated PCT including thyroid cancers, burn, trauma, surgery, pancreatitis, inhalational injury, small cell lung cancer, neuroendocrine tumors, heatstroke, and non-infectious systemic inflammation (1,5). Potential mechanisms include that DKA elevates PCT via systemic inflammation or subclinical underlying pancreatitis. Another additional mechanism may be the influence of worsening renal insufficiency on slowing the elimination of PCT (8). We hypothesize that the elevation of PCT demonstrated in the French study above and in many of our patients who did not have bacterial infection was possible due to increased osmotic shifts including hyperglycemia or due to decreased renal elimination of PCT either due to acute renal insufficiency or the presence of chronic kidney disease. If we had been more successful in obtaining follow-up PCT values, we may have been able to determine the correlation of kidney function parameters including glomerular filtration rate or creatinine clearance with the change in PCT in patients without bacterial infections.
The next steps would be to evaluate the utility of PCT in DKA as part of a treatment algorithm to ensure the safety and efficacy in guiding empiric and definitive antibiotic therapy, while considering the effect of acute or chronic renal insufficiency on PCT values.
Conclusions
While elevations in PCT levels above 0.25 ng/mL may indicate an infection in certain patient populations, in patients with DKA, levels less than 0.25 ng/mL is suggestive for no concomitant bacterial infectious etiology, thus, may not necessitate empiric antibiotics.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-21-38/rc
Data Sharing Statement: Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-21-38/dss
Peer Review File: Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-21-38/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-21-38/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Saint Barnabas Medical Center (IRB protocol 18-29) and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Becker KL, Snider R, Nylen ES. Procalcitonin assay in systemic inflammation, infection, and sepsis: clinical utility and limitations. Crit Care Med 2008;36:941-52. [Crossref] [PubMed]
- Kitabchi AE, Umpierrez GE, Miles JM, et al. Hyperglycemic crises in adult patients with diabetes. Diabetes Care 2009;32:1335-43. [Crossref] [PubMed]
- Sager R, Kutz A, Mueller B, et al. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med 2017;15:15. [Crossref] [PubMed]
- Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev 2017;10:CD007498. [Crossref] [PubMed]
- Jones AE, Fiechtl JF, Brown MD, et al. Procalcitonin test in the diagnosis of bacteremia: a meta-analysis. Ann Emerg Med 2007;50:34-41. [Crossref] [PubMed]
- Ivaska L, Elenius V, Mononen I, et al. Discrepancies between plasma procalcitonin and C-reactive protein levels are common in acute illness. Acta Paediatr 2016;105:508-13. [Crossref] [PubMed]
- Blanchard F, Charbit J. Early sepsis markers in patients admitted to intensive care unit with moderate to severe diabetic ketoacidosis. Ann Intensive Care 2020;10:58. Erratum in: Ann Intensive Care 2020;10:72. [Crossref] [PubMed]
- Meisner M, Lohs T, Huettemann E, et al. The plasma elimination rate and urinary secretion of procalcitonin in patients with normal and impaired renal function. Eur J Anaesthesiol 2001;18:79-87. [Crossref] [PubMed]
Cite this article as: Mistry N, Sobolewski K, Brophy A, Hilden P, Patel A, Vikraman PK. Accuracy of procalcitonin in identifying patients presenting with diabetic ketoacidosis as a result of an infectious etiology. J Lab Precis Med 2022;7:2.