
Hb Agrigente affects haemoglobin A1c laboratory methods: a case report
Introduction
Glycated haemoglobin or haemoglobin A1c (HbA1c) is formed when glucose binds to the N-terminal valine residue of the beta globin chain of Hb A. This attachment undergoes a reversible step called the Maillard Reaction to form a Schiff base (also known as labile HbA1c), before undergoing an irreversible Amadori rearrangement to form HbA1c.
Successful standardisation of laboratory measurements of HbA1c in the early 2000s by the International Federation of Clinical Chemists (IFCC) and Diabetes Control and Complications Trial (DCCT) lead to the recognition of HbA1c as a diagnostic marker for Type 2 diabetes (1,2). However, HbA1c level is affected by a number of conditions such as iron deficiency, vitamin B12 deficiency, chronic kidney disease, conditions affecting red cell turnover and certain haemoglobinopathies; therefore is not recommended as a diagnostic marker for diabetes in patients with these conditions (3).
Here we report a patient with haemoglobin Agrigente (Hb Agrigente) and the analytical dilemma that it poses on laboratory methods of HbA1c in accordance with the CARE reporting checklist (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-21-53/rc).
Case presentation
A 70-year-old woman presented to a general practitioner with 2 days of shortness of breath upon exertion and chest tightness.
Upon physical examination, she was hypertensive with blood pressure (BP) of 166/82, her jugular venous pressure was normal, as was her pulse. She was afebrile, heart sounds were dual with no murmur and chest was clear. Electrocardiogram showed that she was in sinus rhythm there were no acute changes observed during the consultation.
Her past medical history included hypercholesterolemia, left ventricular hypertrophy, diverticular disease, irritable bowel syndrome, osteoarthritis and a previous osteoporotic fracture of the T12. Based on the presenting symptoms, routine pathology tests were ordered, which included a full blood count, electrolytes, kidney and liver function tests, iron studies, lipid profile and a glycated HbA1c.
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and national research committee(s) and with the Helsinki Declaration (as revised in 2013). A verbal informed consent was obtained from the patient for publication of this case report and accompanying images.
Results
Haematology profile was normal and iron studies indicated sufficient plasma iron levels and iron stores. The biochemistry results were unremarkable except for a mildly elevated non-high-density lipoprotein (non-HDL)-cholesterol of 2.9 mmol/L (<2.5 mmol/L). Fasting blood glucose was also normal at 4.9 mmol/L (3.0–5.4 mmol/L).
Cation exchange high performance liquid chromatography (HPLC) (Bio-rad D-100) showed an elevated HbA1c peak of 53.88% (565 mmol/moL) (Figure 1). However, when analysed by HbA1c capillary electrophoresis (CE) (Sebia Capillarys 2 Flexi Piercing), the electropherogram showed a normal HbA1c result of 5.4% (36 mmol/moL) with no variant peaks (Figure 2). This disparity triggered suspicion of a Hb variant co-eluting with Hb A in the CE method. As such, a total glycated haemoglobin by latex agglutination (Siemens DCA Vantage) was obtained which gave a result for HbA1c of 3.6% (15 mmol/moL), this was supported by an enzymatic assay (Abbott Architect) result of 3.8% (18 mmol/moL).
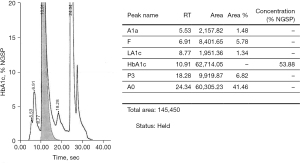
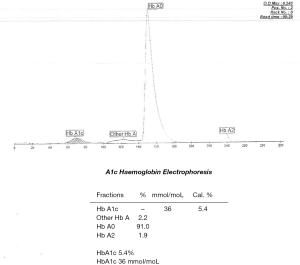
An interference was detected in the measurement of HbA1c and the sample was sent to a reference laboratory for confirmation.
The sample was sent to the haematology department for Hb variant analysis and identification. Automated investigation techniques included the HPLC analyser (Bio-Rad Variant II) which uses the principle of cation exchange HPLC for the separation and determination of the relative percentages of normal and variant haemoglobins, as well as the CE instrument (Sebia Minicap Flex Piercing) for the separation and quantitation of haemoglobin subtypes in alkaline buffer. Additional manual techniques including the electrophoretic separation of haemoglobins in an acid buffered agarose gel and isopropanol stability testing were used to characterise the variant further.
A significant peak was detected in the Hb F window by HPLC that quantitated at 44.8% (Figure 3), there was no separation of the variant by CE (Figure 4), however a haemoglobin band was immobilised in the Hb F region under acid electrophoresis conditions (Figure 5). The isopropanol test was negative indicating the Hb variant has normal stability. These findings suggested the presence of a beta globin gene variant.
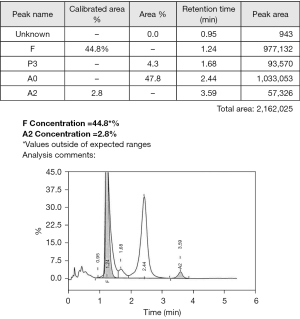


Diagnosis of Hb Agrigente
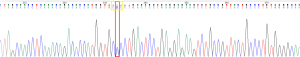
The sample underwent molecular characterisation for identification of the Hb variant by means of DNA extraction from whole blood, and amplification of the beta globin gene using targeted primers following polymerase chain reaction (PCR) principles. Sanger sequencing by CE was used to analyse the purified PCR product, and the resultant DNA sequence was scrutinised for variation from the reference.
A HBB:c.8A>C (pHis2Pro) mutation was detected at codon 2 of the beta globin gene on chromosome 11 (Figure 6). The wild type base was also identified. This mutation is designated Hb Agrigente on the Globin Gene Server Database (4) and the results are consistent with heterozygous inheritance of this beta chain variant.
Haemoglobinopathy screening was recommended in the family.
Due to the interference this variant has on the assays used to determine HbA1c levels, a recommendation was also made to use the oral glucose tolerance test for the diagnosis of diabetes in this patient, and other glycaemic markers such as glycated albumin or fructosamine for future monitoring of glycaemia.
Discussion
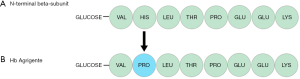
Hb Agrigente is caused by a point mutation at codon 2 of the haemoglobin beta chain molecule resulting in the translation of proline instead of histidine (Figure 7). To our knowledge, there has only been one case published in the literature of Hb Agrigente, which was identified in a 48-year-old diabetic patient of Sicilian origin (5). However, substitution of histidine to proline has also be reported in cases of Hb Marseilles (6), and Hb Long-Island Marseille (7,8) caused by CAT>CCT at codon 2.
The HbA1c of two non-diabetic Hb Long-Island Marseille cases reported in Australia and New Zealand, were abnormally high at 48% (501 mmol/moL) and 45% (468 mmol/moL) respectively. The correlation in findings of elevated HbA1c performed by HPLC in Hb Agrigente, Hb Marseilles and Hb Long-Island Marseilles suggests the effect of these variants on HbA1c lies at the molecular level.
The substitution of amino acid histidine to proline results in a reduction of the beta chain mass from 15,998 Daltons to 15,958 Daltons and the consequential loss of the positively charged ammonium functional group of histidine to form proline, results in a net negative charge from the carboxylic acid (-COOH) (Figure 8).

Both these changes have an effect on the measurement and calculation of HbA1c.
The effects of Hb Agrigente on laboratory assays
Cation exchange HPLC
Cation exchange HPLC uses a negatively charged cation exchange resin, such as a column, to capture positively charged proteins in a buffer with pH at its isoelectric point. The strength of the interactions of the protein and the column will depend on the location of the charge on the molecule as well as functional groups. This allows weak negatively charged proteins e.g., HbA1c to separate first and strong positively charged proteins to separate with changes in the loading buffer pH.
HbA1c is calculated as a ratio of the HbA1c peak over the adult haemoglobin (Hb A) peak.
The change to a net negative charge in Hb Agrigente means the variant separates at a similar retention time as HbA1c, or in some cases, co-elutes and is erroneously classified as the HbA1c peak. Consequently, the % HbA1c appears higher, sometimes above the physiological range.
A solution might be to analyse samples using a longer column and a pH gradient, which would allow for a longer analysis time resulting in better resolution of the two forms.
CE
The principle of CE is based on the migration of haemoglobin fractions by the electrophoretic mobility and particle size of proteins. When proteins are injected into the system at the cathode end under an alkaline pH, they travel through a capillary, commonly made of fused silica, and interact with the negative silica ion cloud on the internal walls of the capillary. Positively charged ions are attracted to the negative silica ions, allowing for small negatively charged ions to migrate towards the anode first in a process called endosmotic or electroosmotic flow. HbA1c is calculated as a ratio of the HbA1c peak over the sum of the HbA1c and Hb A peaks.
The concept of the altered molecular charge described above in HPLC, should be true for CE, in that Hb Agrigente should travel at a similar retention time as HbA1c. However, the absence of an abnormal peak and a “normal” HbA1c peak in the electropherogram (Figure 2) contradict this.
In this case, the differing adult haemoglobin (Hb A) peak in the HPLC method (41.46%) and CE (91.0%) suggests it is mostly likely that the Hb Agrigente has co-eluted with the Hb A in the CE method. Since HbA1c is calculated as a ratio of the HbA1c peak over the Hb A, a lower Hb A as the denominator (41.46%) will give a higher HbA1c result, and conversely, high denominator (91.0%) results in a lower HbA1c.
Enzymatic immunoassay
In enzymatic immunoassays, the glycated N-terminal fructosyl-valine-histidine residue of the beta-chain is cleaved by proteases at the histidine amino acid. The fructosyl-valine-histidine residue reacts with fructosyl peptide oxidase (FPOX) to generate hydrogen peroxide, and produces a quinamine as the Trinder reaction. This is then measured spectrophotometrically (9).
HbA1c is calculated as a ratio of the HbA1c over the total haemoglobin.
The use of genetically modified FPOX enhances the specificity to the fructosyl-valine-histidine peptide of HbA1c, and minimises the interference of fructosyl-lysine peptide which is found in glycated albumin (9).
In this particular case, the effect of the proline amino acid substitution on the glycation of glucose is unknown. However, a possible reason is the change has caused a loss of substrate specificity with FPOX, subsequently leading to a falsely lower HbA1c result (10).
Latex agglutination
Measurement of HbA1c by latex agglutination relies on the binding of HbA1c in the patient sample to antibody-conjugated to a latex particle to inhibit the agglutination, which would otherwise occur in the absence of HbA1c. The assay uses the specificity of the antibody to bind to the glucose of the N-terminal valine of the beta subunit. When the peptide is changed, there is a loss of recognition by the antibody, leading to a falsely low HbA1c level.
Incidence of haemoglobinopathies
Our laboratory is located in a large teaching hospital, providing specialised health care service for more than 700,000 people living in the centre and inner west of Sydney. In addition to our acute services, we are also a reference laboratory and often receive test referrals for HbA1c confirmation from private pathology laboratories.
Our laboratory has a haemoglobin variant detection rate of 2.0% per year (December 2019) and this has progressively increased due to the implementation of a state-wide harmonisation of HbA1c testing and reporting (not published).
In summary, Hb variants may complicate the interpretation of the HbA1c result. Suspicion should be raised when HbA1c results are inconsistent with the patient’s clinical presentation. Failure to detect and investigate the impact of the variant on laboratory measurements of HbA1c has significant clinical implications to the diagnosis and management of diabetes and measurement of alternative markers (fasting glucose or oral glucose tolerance test) may be required.
Not all haemoglobin variants have the same effect but in the case of a patient with heterozygous Hb Agrigente (or Hb Marseille and Hb Long-Island Marseille), the presence of the haemoglobin variant gave a significant elevation of HbA1c by the HPLC method, and a falsely low HbA1c by CE, enzymatic and latex agglutination immunoassay.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-21-53/rc
Peer Review File: Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-21-53/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-21-53/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and national research committee(s) and with the Helsinki Declaration (as revised in 2013). A verbal informed consent was obtained from the patient for publication of this case report and accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mbanya JC, Henry RR, Smith U. Presidents’ statement on WHO recommendation on HbA1c for diabetes diagnosis. Diabetes Res Clin Pract 2011;93:310-1. [Crossref] [PubMed]
- Goodall I. HbA1c standardisation destination—global IFCC Standardisation. How, why, where and when--a tortuous pathway from kit manufacturers, via inter-laboratory lyophilized and whole blood comparisons to designated national comparison schemes. Clin Biochem Rev 2005;26:5-19. [PubMed]
- Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus: abbreviated report of a WHO consultation. Geneva: World Health Organization; 2011.
- Giardine B, Borg J, Viennas E, et al. Updates of the HbVar database of human hemoglobin variants and thalassemia mutations. Nucleic Acids Res 2014;42:D1063-9. [Crossref] [PubMed]
- Philippe MHM, Irenge L, Derelaye I, et al. Abstract 45: Hb Agrigente (Beta(NA2)HIS→PRO). A new beta-globin chain variant found in a diabetic patient. The 6th International Conference on Thalassaemia and the Haemoglobinopathies. Hemoglobin 1997;21:473-9.
- Blouquit Y, Arous N, Lena D, et al. Hb Marseille [α2β2 N methionyl-2 (NA2) His→ Pro]: a new β chain variant having an extended N-terminus. FEBS letters 1984;178:315-8. [Crossref] [PubMed]
- Boi S, Hendy J, Goodall I, et al. First report of HB Long Island-Marseille in Australia—a chance discovery. Hemoglobin 1989;13:515-20. [Crossref] [PubMed]
- Florkowski CM, Walmsley TA, Brennan SO, et al. Haemoglobin Marseille-Long Island and interpretation of HbA1c: which HbA1c result is the “right answer”?. Postgrad Med J 2003;79:174-5. [Crossref] [PubMed]
- Kim S, Miura S, Ferri S, et al. Cumulative effect of amino acid substitution for the development of fructosyl valine-specific fructosyl amine oxidase. Enzyme and Microbial Technology 2009;44:52-6. [Crossref]
- Horiuchi T, Kurokawa T, Saito N. Purification and properties of fructosyl-amino acid oxidase from Corynebacterium sp. 2-4-1. Agricultural and biological chemistry 1989;53:103-10. [Crossref]
Cite this article as: Sherfan J, Oliver S, Ho C, Sullivan DR. Hb Agrigente affects haemoglobin A1c laboratory methods: a case report. J Lab Precis Med 2022;7:7.


