
Comparison of TP53 mutation analysis findings for tar pitch dermatopathy and ionizing radiation-related skin cancer
Introduction
Tar played an important role in the first successful artificial induction of carcinogenesis in rabbits by Yamagiwa et al. (1) and has been identified as an occupational risk factor for various skin diseases since. Among them, tar pitch dermatopathy is mainly of historical importance, and it encompasses both early lesions, such as pigmentation and acne, and late lesions, including acanthoma, Bowen’s disease, and squamous cell carcinoma (2). By the middle of the 20th century, half of all industrial skin cancers were attributed to exposure to tar and pitch in occupations such as coal mining and engineering (3). Coal tar has also been used as a therapeutic regimen for psoriasis as represented by Goeckerman therapy, a combination therapy consisting of crude coal tar and ultraviolet B radiation (4). However, tar pitch dermatopathy itself is very rarely encountered in clinical practice at present because of ecological regulations and occupational health control, and almost no molecular analyses of this type of skin lesion have been performed. On the other hand, x-ray exposure is another historical inducer of occupational skin cancers, typically squamous cell carcinoma (2) occurring in the hands and fingers of radiology professionals. CC to TT tandem transitions and C to T transitions at dipyrimidine sites are well-known mutation signatures for these types of lesions (5).
Even nowadays incidental exposure to environmental or industrial carcinogens brings about a cluster of peculiar cancers and DNA fingerprints (or mutation signature) of these cancer tissues provide our understanding of the reality and novelty of human carcinogenesis (6). Tar-pitch dermatopathy is rare and will be rarer entity, but the findings here, though anecdotal will add some piece of insights on accidental human carcinogenesis.
Methods
We examined the TP53 status in cutaneous lesions related to tar pitch dermatopathy in 4 Japanese patients. Multiple sites including non-tumorous portions were taken from each lesion (Figure S1) and examined. These 4 cases had known occupational exposure to coal tar. For comparison, the same procedures were performed for skin cancers that had occurred on the fingers of 2 patients (3 lesions) with occupational exposure to radiation. The occupational records and diagnoses are shown in the Table 1.
Table 1
| Case | Age at diagnosis/sex | Occupation [location of lesion(s)] | Duration of exposure (years) | Pathology | TP53 mutation (exons 4 to 8) | Outcome |
|---|---|---|---|---|---|---|
| 1 | Late 70s/M | Fisherman (multiple sites: foot, leg, and arm) | 50 | Squamous cell carcinoma | c.[715A>G(;)503A>C]; p.[(N239D(;)H168P)] | Unknown |
| 2 | 50/M | Fisherman (buttock) | 47 | Squamous cell carcinoma | None | Unknown |
| 3 | 61/M | Iron Foundry (left knee) | 39 | Bowen’s disease | None | No recurrence |
| 4# | 77/M | Iron Foundry (left hand) | 30 | Squamous cell carcinoma | c.625A>T; p.(R209*) (note) | Death due to metastasis |
| 5 | 79/M | Radiology engineer (finger) | 22 | Squamous cell carcinoma | c. [224delC(;) 470delT]; p.[(P75Lfs*48(;)V157Afs*13)] (codon 75 CCT>CT) (codon 157 GTC>GC) | Alive |
| 6 | 47/M | Orthopedic surgeon (finger) | 25 | Squamous cell carcinoma | None | Alive |
#, this mutation has also been found in a small cell lung cancer cell line (H2141). M, male.
Using samples stored in pathology archives, DNA was extracted from multiple sites of each lesion. Macroscopically and manually multiple portions were taken and DNA analysis were conducted (Figure S1). PCR amplification was not successful for some of the DNA samples from these multiple sites in an individual case. Finally, confirmative sequences could be obtained in 8 lesions in 4 cases. For a sample in which PCR amplification was successful, ordinary Sanger sequencing was performed according to previously described methods (7). Primers covering exons 4 to 8 of TP53 were used and are available upon request. The entire procedure was repeated more than twice to confirm the mutation findings.
An immunohistological study was performed using a standard method, and the clone DO-7 (Dako Japan, Tokyo) was used to reveal the distribution of p53 protein in the tissues (8). This DO-7 antibody identifies both the wild type and the mutated form of the p53 protein and some mutant p53s has longer half-life in the cells. Wider varieties of p53 are known currently but the majority of missense mutants are detectable by this antibody and DO-7 is widely used in practice, too (9).
The numbers of the cases and clones were few and statistical interpretation was not done.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board of Hamamatsu University School of Medicine (No. 20-011). Informed consents were not required from the participants, because the study was conducted by using residual pathological archives in anonymous way and no intervention was included in any part of the study.
Results
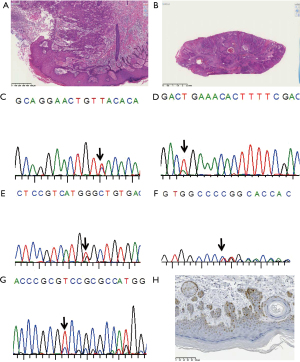
Base substitution-type mutations (missense and nonsense types) were found in skin cancer arising from tar pitch dermatopathy. The histopathology of the cases and sequence graphs are shown in Figure 1. In case 1 (Figure 1A) and case 2 (Figure 1B) both exhibit squamous cell carcinoma in dermatopathy. Representative sequenograms are shown in Figure 1C-1G. While the mutation c.715A>G was identified (Figure 1C) in one portion, and an additional c.503A>C mutation (Figure 1E) was found in another portion (double mutation in this portion), no mutation was found in case 2 though almost similar histology. In case 4, the mutation c.625A>T was identified (Figure 1D). This mutation generates a stop codon, and the cancer specimen was negative for immunoreactivity to p53 protein (data not shown). In contrast, different deletion-type mutations (Figure 1F,1G) were found at different sites in one of the two cases with occupational radiation exposure. Figure 1H is p53 protein immunostaining in the case 1. Nearly clonal p53 protein staining is noticed.
Discussion
Even in the era of massive parallel sequencing, TP53 is regarded as a gene that very frequently exhibits somatic mutations in most common tumors; thus, the mutation spectrum of TP53 can provide etiological clues regarding human carcinogenesis (10). Tar contains many carcinogens, with benzo[a]pyrene being one of the most potent and extensively investigated substances. Benzo[a]pyrene is metabolized to benzo[a]pyrene-7,8-diol-9,10-epoxide (BPDE), which binds to guanine covalently and induces mispairing and mutation to thymine at that guanine position (11). This pathway agrees with mutation spectrum findings in human tobacco-associated lung cancer (12). Unexpectedly, the coal tar-related skin cancers examined in this study did not harbor any typical G to T transversions in TP53 within the region spanning exons 4 to 8, though multiple sites were examined. The reason for this unexpected finding is unknown.
On the other hand, the somatic c.625A>T (p.R209*) mutation, found in the present study, has been recorded in 18 cases in the IARC database (13). Interestingly, 15 of these cases were cancers that were strongly correlated with tobacco smoking (lung, esophagus, urinary tract, hypopharynx, and oropharynx). The other three tumors were soft tissue leiomyosarcoma, breast, and ovarian cancer. A germline mutation of this type has also been recorded. None of the presently reported cases had a family history of cancer, and wild-type TP53 was detected in non-tumorous skin samples.
Fifty-two cases of somatic c.715A>G mutations have been included in the IARC database, 19 of which were tobacco-related cancers. Meanwhile, the c.503A>C mutation has been identified in 14 entries, 4 of which were tobacco-related cancers. No skin cancers with the mutations found in the present analysis have been included in the IARC TP53 database (R20 release) (13). The presently reported mutation type suggests that these mutations are caused by environmental factors other than sunlight (the commonest cause of skin cancer), such as tar-pitch-related oxidative damage including adenine deamination (14).
In skin cancer, which is most commonly related to UV or sunlight exposure, the mutation spectrum favors CC to TT changes via the formation of pyrimidine dimers and C to T transitions at dipyrimidine sites (15). Actually, as shown for comparison, the two novel mutations found in one of our cases with a strong background of occupational ionizing radiation occurred at CC and CT positions (codon 75 was CCT, codon 157 was CTG, case 5), as would be generally expected, and our hypothesis seems to be reasonable.
Takata et al. (16) found 9 mutations in Japanese basal cell carcinoma, 2 of which were G to T transversions, but no mutations from adenine were listed. They did not mention any particular exposure history for these subjects. We cannot draw any definitive conclusions based on the small number of cases in the present study, but the situation in tar pitch dermatopathy appears to be quite different from that in other skin cancers and to be more similar to that occurring in tobacco-related cancer, though typical benzo[a]pyrene-induced mutations were not identified. In a recent study, anti-BPDE-DNA adducts were measured in psoriasis patients who had received Goeckerman therapy (17), but straightforward correlations between the antibody level and the exposure attributes were not found. Thus, the effect of tar-pitch is not always and not simply that of benzo[a]pyrene.
This study has inevitable limitations. The numbers of the cases are small and anecdotal. The samples are old archives, thus, extensive sequence analysis using next generation sequencing was not possible. We did make only manual dissection to isolate epidermal tissue with and without malignant or premalignant changes, thus single cell level analysis which combined immunohistochemical detection of p53 protein with sequence results was not done.
Very recently, massive parallel sequencing has identified somatic mutations in normal-looking skin; thus, our observations reported here may represent a final phase in the clonal evolution of squamous cell carcinoma (18). Although multiple sites were analyzed, PCR was not always successful, probably because the fixation and storage condition were uncontrollable. Further elucidation of the steps in clonal evolution in these particular settings is needed, and a molecular signature study of skin cancers with specific occupational incidences (when not avoidable) may provide a clue to an improved understanding of human skin carcinogenesis. Tar-Pitch dermatopathy and other accidental occurrences of human cancer may happen someday. Even for such an incidence careful preservation and preparation of specimens for future research is necessary.
Acknowledgments
Funding: We greatly appreciate the grants from the Ministry of Health, Labour and Welfare (No. 21-1,10103838), the Ministry of Education, Culture, Sports, Science and Technology (MEXT) (Nos. 221S0001, 26670187), the Princess Takamatsu Cancer Research Fund, and the Smoking Research Foundation.
Footnote
Data Sharing Statement: Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-21-69/dss
Peer Review File: Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-21-69/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-21-69/coif). HS serves as an unpaid editorial board member of Journal of Laboratory and Precision Medicine from October 2021 to September 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board of Hamamatsu University School of Medicine (No. 20-011). Informed consents were not required from the participants, because the study was conducted by using residual pathological archives in anonymous way and no intervention was included in any part of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yamagiwa K, Ichikawa K. Exprimentele Studie über die Pathogenese der Epithelialgeschwülste. Mitteilungen Med Fakultät Kaiserl Univ Tokyo 1915;15:295-344.
- Gawkrodger DJ. Occupational skin cancers. Occup Med (Lond) 2004;54:458-63. [Crossref] [PubMed]
- Voelter-Mahlknecht S, Scheriau R, Zwahr G, et al. Skin tumors among employees of a tar refinery: the current data and their implications. Int Arch Occup Environ Health 2007;80:485-95. [Crossref] [PubMed]
- COLE HN Jr. Goeckerman therapy in the management of common dermatoses. Arch Dermatol 1959;80:788-91. [Crossref] [PubMed]
- Daya-Grosjean L, Dumaz N, Sarasin A. The specificity of p53 mutation spectra in sunlight induced human cancers. J Photochem Photobiol B 1995;28:115-24. [Crossref] [PubMed]
- Mimaki S, Totsuka Y, Suzuki Y, et al. Hypermutation and unique mutational signatures of occupational cholangiocarcinoma in printing workers exposed to haloalkanes. Carcinogenesis 2016;37:817-26. [Crossref] [PubMed]
- Yamada H, Shinmura K, Okudela K, et al. Identification and characterization of a novel germ line p53 mutation in familial gastric cancer in the Japanese population. Carcinogenesis 2007;28:2013-8. [Crossref] [PubMed]
- Igarashi H, Sugimura H, Maruyama K, et al. Alteration of immunoreactivity by hydrated autoclaving, microwave treatment, and simple heating of paraffin-embedded tissue sections. APMIS 1994;102:295-307. [Crossref] [PubMed]
- Sawada K, Momose S, Kawano R, et al. Immunohistochemical staining patterns of p53 predict the mutational status of TP53 in oral epithelial dysplasia. Mod Pathol 2022;35:177-85. [Crossref] [PubMed]
- Harris CC, Hollstein M. Clinical implications of the p53 tumor-suppressor gene. N Engl J Med 1993;329:1318-27. [Crossref] [PubMed]
- Weinberg RA. The Biology of Cancer, second edition. New York and London: Garland Science, 2014.
- Rodin SN, Rodin AS. Human lung cancer and p53: the interplay between mutagenesis and selection. Proc Natl Acad Sci U S A 2000;97:12244-9. [Crossref] [PubMed]
- Bouaoun L, Sonkin D, Ardin M, et al. TP53 Variations in Human Cancers: New Lessons from the IARC TP53 Database and Genomics Data. Hum Mutat 2016;37:865-76. [Crossref] [PubMed]
- Spencer JP, Whiteman M, Jenner A, et al. Nitrite-induced deamination and hypochlorite-induced oxidation of DNA in intact human respiratory tract epithelial cells. Free Radic Biol Med 2000;28:1039-50. [Crossref] [PubMed]
- Brash DE. UV signature mutations. Photochem Photobiol 2015;91:15-26. [Crossref] [PubMed]
- Takata M, Rehman I, Rees JL. p53 mutation spectrum in Japanese Bowen's disease suggests a role for mutagens other than ultraviolet light. Int J Cancer 1997;71:370-2. [Crossref] [PubMed]
- Borska L, Andrys C, Krejsek J, et al. Serum level of antibody against benzoapyrene-7,8-diol-9,10-epoxide-DNA adducts in people dermally exposed to PAHs. J Immunol Res 2014;2014:834389. [Crossref] [PubMed]
- Martincorena I, Roshan A, Gerstung M, et al. Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science 2015;348:880-6. [Crossref] [PubMed]
Cite this article as: Ishino K, Yamada H, Kahyo T, Hino R, Nakamura M, Shimajiri S, Hisaoka M, Kasami M, Tokura Y, Sugimura H. Comparison of TP53 mutation analysis findings for tar pitch dermatopathy and ionizing radiation-related skin cancer. J Lab Precis Med 2022;7:11.


