
The usefulness of glycated albumin in patients with diabetes and renal disease: a scoping review
Introduction
Chronic kidney disease (CKD) is considered a public health problem and it is one of the main complications in patients with diabetes mellitus (DM). CKD may be diagnosed by persistent high urinary albumin excretion (albuminuria), low estimated glomerular filtration rate (eGFR), or other signs of kidney damage (1). Around 20% to 40% of DM patients develop kidney disease, called diabetes renal disease (DRD), with or without overt proteinuria (2). DRD can progress to end-stage renal disease (ESRD) requiring dialysis or kidney transplantation. Also, DRD significantly increases cardiovascular risk and health care costs. An appropriate glycaemic control decreases the rate of progression to renal failure in these patients; therefore good control of glycaemia is important since earlier stages of CKD (2).
Glycated haemoglobin (HbA1c) is the standard test for glycaemic monitoring in patients with DM (1). However, this assay is influenced by several pathophysiological conditions that become this test unreliable in patients with CKD, such as anaemia, uraemia, erythropoietin use and treatment by haemodialysis (HD), and its interpretation is difficult (3-5). Glycated albumin (GA) is a promising test with increasing interest in the last years as a potential marker of glycaemic control (6). Biochemically, the glycation of albumin to generate GA is similar to that of haemoglobin to yield HbA1c and it has been proposed as an alternative marker of glycaemic control in conditions wherein erythrocytes lifespan is altered or other condition that affects HbA1c (6). Some data reported that GA may be superior to HbA1c in assessing blood glucose control in diabetes patients with advanced CKD (7) due to GA it is not influenced by anaemia or treatments such as erythropoietin or HD. Also, GA is a short-term glycaemic index and indicates more rapidly variations of glycaemia than HbA1c.
In this way, the objective of this review was to summarize the evidence in the literature about the usefulness and limitations of GA as a glycaemic marker in patients with diabetes with CKD. We present the following article in accordance with the PRISMA Scoping Review reporting checklist (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-22-2/rc).
GA: a quick overview
GA is derived from non-enzymatic glycation between albumin, the most abundant protein in plasma, and glucose. Glycation is a physiological process that occurs when an N-terminal amino acid residue binds to sugar and produces a chemical product called “fructosamine”. Therefore, GA is a specific type of fructosamine that represents about 80% of the total of glycations in plasma (6). Although glycation is a natural process, it modifies the structure of albumin, leading to a reduction in the antioxidant activity of the protein and affecting its binding properties (8). Besides, advanced glycation stages of albumin induce the formation of advanced glycation end-products (AGEs), which account for additional oxidative events, related to several health complications (9).
Serum albumin is the most sensitive protein to glycation. This is mainly due to its high concentration in the body and its turnover is smaller when compared to other proteins, including haemoglobin (21 days against 120 days) (9). According, GA provides the glycaemic balance over 3 weeks, and it is considered a short-term biomarker for DM (8). When compared to HbA1c, the glycaemic marker recommended for DM monitoring and diagnosis by international consensus (1), GA has some advantages. GA is not affected by haemoglobin turnover, therefore, measurement of GA is not influenced by anaemia and iron deficiency (10), and it is also a good predictor of chronic complications in DM. Then, GA has been accepted as an alternative biomarker of glycaemic control when HbA1c is not reliable (6).
There are several methods proposed for the quantification of GA, as colorimetry, chromatography, immunoassay, and mass spectrometry. In 2002 a new enzymatic method for GA measurement was described (11) and, posteriorly, launched into the market. This method (Lucica GA-L®, Asahi Kasei Pharma Corporation, Tokyo, Japan) employees an albumin-specific proteinase, which yields a simple, rapid, accurate and easily automatized technique (12,13). After Lucica GA-L®, other enzymatic assays from different manufacturers have been launched, but with similar performance (14-16).
Although the GA has become highly studied, and its safety and efficiency has been scientifically evaluated, it is still few employed for clinical practice in countries other than Asians (17). One limitation for the applicability of GA in the routine of clinical labs may be the higher cost when compared to HbA1c. We have compared two different assays for GA and the price per test was around $4 to $6, in contrast with HbA1C test that is around $2 to $3 in Brazil (6,15). However, this scenery is likely to change in a near future. Studies show that reference levels of GA vary from approximately 10% to 18% (18), with a considerable low biological variation (19).
However, there are some conditions that may affect its levels and lead to misinterpretation. For instance, in albumin catabolism increases, as in nephrotic syndrome and hyperthyroidism, wherein lower GA values do not accurately reflect blood glucose concentrations (9). Nephrotic-range proteinuria decreases GA levels independent of the glycaemic status in patients with diabetes with CKD (20). GA is also reported to serve as a safe indicator of glycaemic control in patients with diabetes on dialysis, once the influence of albumin leakage induced by HD on GA levels was reported to be practically negligible (21). Overweight and obese individuals also present a negative correlation with GA, probably because of the chronic inflammation involved, which increases albumin turnover (6,15). In HD patients with DM, GA exhibited inverse correlations with BMI, total lean mass, total fat mass, and truncal fat mass (22). On the other hand, GA values are reported to be higher in conditions in which albumin metabolism is reduced, such as in liver cirrhosis and hypothyroidism (9). Age can also influence GA levels, whereas the effect of gender is not clear (15). Children show higher GA than adults and among adults at older ages, particularly for men, GA seems to be higher (8). In non-diabetic ESRD patients, GA values were influenced by age and nutritional status independent of glycaemia (23). It is well known that there are ethnic differences in HbA1c levels. However, information about the ethnic influence on GA levels is scarce since the majority of studies were carried out in Asian (18).
Several studies showed that glycaemic control, measured by HbA1c or GA, is an independent predictor of clinical outcome and mortality in people with DM (24-29) and targeting lower glycaemic levels has been proven to reduce risks of microvascular DM complications and, in some studies, also macrovascular DM complications (30-34). All these situations must be considered when interpreting markers of glycaemia results.
Review search strategy
This is a scoping review with a systematic search of literature. We searched PubMed (MEDLINE) for reports published up to May 2021 using the search terms related to DM, GA and renal disease combined. Details of all search terms are presented in Table S1. From the papers retrieved, a manual search of their references was conducted. Duplicate were removed and the remaining reports were assessed for eligibility, regardless of the language.
Selection criteria
Inclusion criteria were: (I) cross-sectional or cohort studies that assessed the GA as glycaemic marker in patients with diabetes with renal disease; (II) studies that analysed specifically GA by enzymatic assays. Exclusion criteria were: (I) study that was not performed in patients with diabetes with renal disease; (II) review articles; (III) editorial/comments/letters/case reports; (IV) basic research articles; (V) drug clinical trial reports. Two independent reviewers (FCC and JLC) decided for studies inclusion based upon eligibility criteria. First, we screened the titles of all search results to identify potentially relevant articles. Next, we reviewed the abstracts of these studies to define their relevance, and once judged to be relevant, reviewed the full text of the studies. Finally, we analysed each article, ascertained whether the article was qualified for inclusion and performed findings summary from all included reports. Any disagreements concerning study eligibility or data interpretation were resolved through discussion between reviewers.
Study characteristics
The search strategy identified 311 records, of which 119 were assessed for eligibility. We then excluded 58 papers (27 did not meet the research question; 9 were editorial, comment, letter, case report or book chapter; 8 were drugs clinical trial reports; 5 were basic research studies; 4 were not available in full text, 2 presented missing data and 3 was duplicate). Sixty-one studies met our inclusion criteria and were included in the qualitative synthesis of this review. These studies were related to glycaemic control (35-59), outcomes in patients with diabetes in dialysis (60-75), outcomes in patients with diabetes without overt renal disease (54,58,76-89), technical aspects of GA measurement (12), interfering factors in GA analysis (20-23) and diabetes mellitus post-transplant (DMPT) (90). The search strategy is depicted in Figure 1 and Table 1.
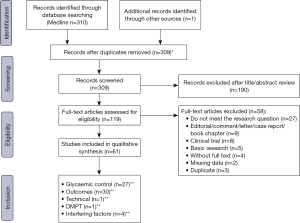
Table 1
| Items | Specification |
|---|---|
| Date of search (specified to date, month and year) | 21st May 2021 |
| Databases and other sources searched | PubMed (MEDLINE) |
| Search terms used (including MeSH and free text search terms and filters) | Diabetes mellitus, glycated albumin, and renal disease combined (see Table S1 for details) |
| Timeframe | Reports published up to May 2021 |
| Inclusion and exclusion criteria (study type, language restrictions etc.) | Inclusion criteria: (I) cross-sectional or cohort studies that assessed the GA as glycaemic marker in patients with diabetes with renal disease; (II) studies that analysed specifically GA by enzymatic assays |
| Exclusion criteria: (I) study that was not performed in patients with diabetes with renal disease; (II) review articles; (III) editorial/comments/letters/case reports; (IV) basic research articles; (V) drug clinical trial reports | |
| Selection process (who conducted the selection, whether it was conducted independently, how consensus was obtained, etc.) | Two independent reviewers (FCC and JLC) decided for studies inclusion based upon eligibility criteria. Any disagreements concerning study eligibility or data interpretation were resolved through discussion between reviewers |
GA, glycated albumin.
GA and glycaemic control in patients with DM and CKD
The goals and plans of treatment for DM are to prevent or delay complications and optimize quality of life, and in CKD patients are not different. Several studies showed that glycaemic control is an independent predictor of clinical outcome and mortality in people with DM and CKD (24-29). Thus, maintaining blood glucose at recommended levels is essential for people with DM and CKD. However, CKD alters glycaemic control, the results of the HbA1c test, and the excretion of antidiabetic medications. The effects of CKD and dialysis can make blood glucose levels fluctuate widely, placing patients at risk of hypoglycaemia.
Self-monitoring of blood glucose (SMBG), continuous glucose monitoring (CGM), and HbA1c measurement are recommended for people with diabetes without ESRD by international guideline (1). For daily glycaemic monitoring, CGM and SMBG are frequently used but they are relatively high-cost options to assess real-time blood glucose. For these reasons, CGM and SMBG, even for people with DM and CKD, are not yet widely used but recommended to improve glycaemic control when anti-hyperglycaemic therapies are associated with the risk of hypoglycaemia (such as insulin) are used (2). In accordance with the recommendations of the Kidney Disease Improving Global Outcomes (KDIGO) (2) glycaemic control in patients with DM and CKD should be based on HbA1c measurements. This recommendation is motivated by the fact that in randomized control trials, targeting lower HbA1c values has been proven to reduce risks of microvascular DM complications and, in some studies, also macrovascular DM complications (i.e., cardiovascular events) (30-34) However, in people with DM and CKD HbA1c results may be affected by several factors (2,3). Thus, mainly in ESRD, HbA1c levels should be interpreted with caution (2) and there is an interest in an alternative marker to HbA1c. GA has been proposed as a candidate for alternative long-term glycaemic monitoring (6).
From our initial literature search 26 studies that evaluated the correlations of GA and HbA1c with blood glucose measures among patients with CKD and/or HD were selected and included in this section of our review (35-52,54-60). One additional study (53) with the same objective was identified during review of these studies. Table 2 shows the main characteristics and findings of these studies. The majority of studies reported that GA correlates with HbA1c in patients with CKD, including patients on dialysis (36-38,47,50,51,53,54,57). The association of GA and HbA1c with measures of glycaemia [fasting plasma glucose (FPG), random plasma glucose, or average blood glucose by SMBG or CGM] is similar and suggest that GA may be a useful substitute for HbA1c or as a complement for monitoring glucose control. However, the associations presented inconsistencies and varied widely from strong to none association. Some studies found that the correlation of GA with blood glucose was stronger than the correlation of HbA1c with blood glucose (35,36,39,41,42,46,48,49,53-57). Nevertheless, other studies reported worse correlations of GA with glycaemia than correlations of HbA1c with glycaemia (37,38,40,43-45,47,50-52,59,60). The study of Yajima et al. (55) included only patients on peritoneal dialysis and reported no correlation between CGM and GA (45), while another study found no correlation in a group of patients on HD for a period of less than 6 months.
Table 2
| Author, year publication | Study location | Sample size (n) | Age‡ (years) | CKD stage* | eGFRCKD-EPI‡ (mL/min/1.73 m2) | Measure of glucose | Correlation (R) | ||
|---|---|---|---|---|---|---|---|---|---|
| GA with HbA1c | GA with glycaemia | HbA1c with glycaemia | |||||||
| Chujo K, 2006 | Japan | 49 | 63.9±13.1 | Pre-dialysis G5 | NA | SMBG | NA | 0.560 | 0.470 |
| 37 | 64.4±11.1 | G5 | HD | SMBG | NA | 0.500 | 0.420 | ||
| Inaba M, 2007 | Japan | 538 | NA | G5 | HD | RPG | 0.777 | 0.539 | 0.520 |
| Nagayama H, 2009 | Japan | 23 | 61.3±1.83 | G5 | HD | OGTT | 0.728 | 0.660 | 0.665 |
| Uzu T, 2009 | Japan | 87 | NA | G5 | HD | RPG | 0.754 | 0.520 | 0.539 |
| Freedman BI, 2010 | USA | 470: 415 HD/55 PD | HD: 63.0±12.3; PD: 58.1±13.4 | G5 | HD, PD | RPG | NA | 0.390 | 0.380 |
| Tajiri Y, 2010 | Japan | 112 | 63.6±14.3 | G3–5 | 24.4±13.7 | MPG | NA | 0.450 | 0.730 |
| 97 | 64.2±12.0 | G5 | HD | MPG | NA | 0.750 | 0.590 | ||
| Kim JK, 2012 | Korea | 185: 108 HD/77 PD | NA | G5 | HD, PD | SMBG | NA | 0.700 | 0.500 |
| Vos FE, 2012 | New Zealand | 25: 13 non-HD/7 HD/5 PD | 60.2 [32–79] | G4, G5 | 18.0 [12.0–30.0]† | CGM | NA | 0.540 | 0.380 NS |
| Chen FK, 2013 | China | 88 | 61.0±13.0 | G5 | HD | MPG | NA | 0.380 | 0.511 |
| Konya J, 2013 | UK | 15 | 70.0 [62–75] | G3B, G4 | 15–44 | MPG | NA | 0.100–0.670 | 0.700–0.880 |
| Lee SY, 2013 | Taiwan | 25 | 59.0±13.0 | G5 | PD | CGM | NA | −0.260 NS | 0.510 |
| Sany D, 2013 | Egypt | 25 | 43.8±9.0 | CKD without dialysis; CKD stage NA | NA | RPG | 0.650 | 0.580 | 0.560 |
| 25 | 43.8±11.0 | G5 | HD | RPG | 0.630 | 0.970 | NA- | ||
| 25 | 46.8±7.0 | G5 | HD | RPG | 0.700 | 0.540 | 0.510 | ||
| Harada K, 2014 | Japan | 28 | 57.0±10.8 | G1 | 77.6±13.3 | RPG | 0.757 | 0.670 | 0.739 |
| 69 | 67.3±10.1 | G2 | 44.2±8.4 | RPG | 0.710 | 0.556 | 0.561 | ||
| 42 | 68.3±11.3 | G3 | 18.2±8.0 | RPG | 0.226 | 0.361 | 0.289 | ||
| Fukami K, 2015 | Japan | 30 | 63.0±12.0 | G4, G5 | 9.3±6.7 | MPG | NA | 0.41 | 0.240 ns |
| Kim IY, 2015 | South Korea | 97 | 65.2±11.5 | G4, G5 | 23.5±15.7 | FPG | NA | 0.671 | 0.544 |
| 24 | 63.4±15.1 | G1, G2 | 82.7±16.2 | FPG | NA | 0.872 | 0.892 | ||
| Williams ME, 2015 | USA | 1,758: 1,476 HD/282 PD | 62.1±12.6 | G5 | HD, PD | RPG | 0.770 | 0.630 | 0.690 |
| Hayashi A, 2016 | Japan | 41 | 60.2±11.7 | G5 | HD | CGM | 0.610 | 0.420 | 0.590 |
| Kobayashi H, 2016 | Japan | 20 | 59.6±9.5 | G5 | PD | PG | NA | 0.166 | 0.620 |
| 20 | 58.6±7.4 | G5 | HD | PG | NA | 0.121 | 0.670 | ||
| Tsuruta Y, 2016 | Japan | 46 | 66.3±11.4 | G5 | HD | MBG | 0.697 | 0.385 | 0.363 |
| Wang N, 2017 | China | 71 | 66.0±11.0 | Mainly G3, G4 | G3: 30–59; G4: 15–29 | MBG | 0.556 | 0.628 | 0.537 |
| Yajima T, 2017 | Japan | 16 | 70.8±8.1 | G5 | Shor-time HD (duration <6 months) | CGM | NA | 0.340 NS | 0.100 NS |
| 15 | 70.8±8.1 | G5 | Long-time HD (duration >6 months) | CGM | NA | 0.554 | 0.546 | ||
| Divani M, 2018 | Greece | 37 | 62.0±17.2 | G5 | HD | CGM | NA | 0.884 | 0.694 |
| Jung M, 2018 | USA | 724 | 74.9 (4.7) | G1 | >60 | FPG | 0.690 | 0.520 | 0.650 |
| 464 | 76.4 (5.1) | G2 | 45–60 | FPG | 0.720 | 0.490 | 0.630 | ||
| 268 | 77.2 (5.4) | G3 | 30–45 | FPG | 0.700 | 0.390 | 0.520 | ||
| 209 | 78.3 (5.6) | G4 | ≤30 | FPG | 0.70 | 0.36 | 0.48 | ||
| Bellia C, 2019 | Italy | 81 | 67.0±14.0 | G4, G5 | 21.0 [13–26] | FPG | NA | 0.41 | 0.42 |
| Zelnick LR, 2020 | USA | 104 | 68.0±10.0 | G1, G2, G3 | 38.0±14; 83.0±11 | CGM | NA | 0.93 | 0.95 |
*, CKD stage was mainly determined by eGFR; ‡, data are expressed as mean ± SD or median [interquartile range]; †, average including non-dialysis patients only. GA, glycated albumin; HbA1c, glycated haemoglobin; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; NA, not available; HD, haemodialysis; PD, peritoneal dialysis; SMBG, self-monitoring of blood glucose; RPG, random plasma/serum glucose; OGTT, oral glucose tolerance test; MPG, mean plasma glucose; CGM, continuous glucose monitoring; FPG, fasting plasma glucose; PG, plasma glucose; NS, not significant.
Considering the inconsistency of findings in available observational studies, the lack of clinical trials based on GA in patients with CKD, and GA is not readily available for use, it might be inappropriate to dispense HbA1c in favour of GA. Besides, the results regarding the influence of stage of CKD on the association of GA with glycaemia also varied, although most studies report no influence, other studies report the influence of CKD severity, including those treated by dialysis (46,47,52,55,57,58). Furthermore, GA to monitor glycaemic control in patients with CKD should be interpreted with caution, since those patients may present diminished serum albumin due to massive proteinuria, malnutrition, or peritoneal dialysis. Studies in patients with CKD found that GA was correlated with albumin, and the GA level can be falsely low in hypoalbuminemia (36,41,47,48). Therefore, some studies to overcome this inaccuracy suggested serum albumin-adjusted GA. In these studies, serum albumin-adjusted GA was not affected by protein loss or renal anaemia, represented glycaemic excursion and glycaemic control better than GA alone or HbA1c in patients with CKD (48,55,89). Considering the limited data concerning serum albumin-adjusted GA, future studies about glycaemic control in patients with CKD should explore and validate this new parameter.
GA and outcomes
Chronic hyperglycaemia is the main cause of diabetes complications and glycaemic control is essential to diabetes management (1). It is very well stablished by large prospective randomized controlled trials that good glycaemic control is associated with reduction of development and progression of retinopathy, neuropathy, and diabetic kidney disease, common diabetes complications, both in type 1 and type 2 DM. Traditionally, HbA1c is the marker of choice to measure glycaemic control, therefore reduction in HbA1c levels are associated with reduction in rates of development and progression of complications (1).
Since the early 2000’s, when GA test became available (11), several studies analysed the association of GA levels and diabetes complications. Recently, a meta-analysis (91) that included 25,932 patients with diabetes undergoing dialysis from 12 studies, with follow-up up to 11 years, showed that higher GA levels were associated with the risk of all-cause mortality in dialysis patients with DM regardless of the type of dialysis, whereas higher GA was not associated with cardiovascular mortality. However, these results were modest and showed a small effect size. The studies included in this meta-analysis were very heterogeneous. The authors related limitations to their study such as the presence of many CKD-related variables that may affect GA levels, lack of randomized controlled trials, inclusion of small observational and cross-sectional studies and that most studies were carried out in Asian countries. All these factors would difficult the applicability of the results to all populations (91).
In this review, our systematic search identified 30 articles that evaluated the association of GA with DM complications in patients with dialysis treatment (54,58,61-75) and without dialysis treatment (76-89). Most of these studies were carried out in Asian countries (54,58,76,77,80-87) and only two studies were conducted by American centres (78,79).
Fifteen studies analysed the association of GA with complications in patients with diabetes undergoing dialysis treatment (54,58,61-75). The characteristics and main findings of these reports are described in Table 3. The majority of these studies reported the relationship of GA levels with mortality and showed that higher GA levels are related to higher risk for all-cause mortality and also for cardiovascular disease (CVD) mortality in patients with diabetes under dialysis treatment (61,62,65,67-75). The degree of these interactions varied from weak to modest in all studies. Twelve of these studies were included in the recent meta-analysis by Copur et al. (91). Kumeda et al. showed that increased GA values were associated with increased arterial stiffening (63) and Yamada et al. reported that GA values were associated with the presence of peripheral vascular calcification (64). Murea et al. reported that improved glycaemic control based on GA predicted cardiovascular-related hospitalizations and hospital length of stay in patients with diabetes on HD treatment (66). Most of these studies were carried out in Japan (61-64,68,70,72-75), 3 in American centres (65-67), one in Taiwan (69) and one in Germany (71).
Table 3
| Author, year publication | Study location | Type of study | Sample size (N) | Main findings |
|---|---|---|---|---|
| Okada T, 2007# | Japan | Observational. Follow-up: mean 35.0 months (2–48 months) | 78 DM2 | GA levels, at initiation of dialysis or on chronic dialysis, did not predicted mortality. Poor glycaemic control, identified by high GA levels (≥23.0%), showed association with the development of CVD (HR: 3.25; 95% CI: 1.04–10.19; P=0.04) |
| Fukuoka K, 2008# | Japan | Observational. Follow-up: mean 47.7 months (0–10 years) | 98 DM | The cumulative survival rate of GA <29% group was significantly higher than GA ≥29% group (P=0.034; log-rank test). After adjustment, High GA (GA ≥29%) was a significant predictor of survival (HR: 1.042 per 1.0% increment of GA; 95% CI: 1.014–1.070; P<0.05), and cardiovascular death (HR: 2.971; 95% CI: 1.064–8.298; P=0.038) |
| Kumeda Y, 2008 | Japan | Cross-sectional case-control | 134 DM2; 158 without DM | In diabetic patients increased GA values were associated with increased arterial stiffening |
| Yamada S, 2008 | Japan | Cross-sectional | 49 DM2 | GA and HD duration were significantly associated with the presence of peripheral vascular calcification. When GA was replaced by HbA1c in the same model, HbA1c failed to show a significant association |
| Freedman BI, 2011#* | USA | Observational Follow-up: median 27.2 months (0.56–27.8 months) |
444 DM | GA accurately predicts the risk of death and hospitalizations in patients with DM and ESRD |
| Murea M, 2012#* | USA | Observational. Follow-up: 2.33 years | 444 DM | Improved glycaemic control based on GA predicted cardiovascular-related hospitalizations (HR: 1.32; 95% CI: 1.11–1.57; P=0.002 at 17 days; HR: 1.21; P=0.02 at 30 days), and also predicted hospital length of stay (HR: 1.18; 95% CI: 1.01–1.39; P=0.03) |
| Shafi T, 2013# | USA | Prospective cohort. Follow-up: median of 3.5 years | 287 DM; 216 without DM | GA was associated with all-cause mortality (adjusted HR per doubling of the biomarker 1.40; 95% CI: 1.09–1.80; P=0.008), and with CVD mortality (HR: 1.55; 95% CI: 1.09–2.21; P=0.02) |
| Isshiki K, 2014# | Japan | Observational. Follow-up: median 36.0 months (3–36 months) | 90 DM2 | GA predicted mortality (HR: 1.143 per 1% increase in GA; 95% CI: 1.011–1.292; P=0.033). The cumulative survival rate was significantly greater in patients with GA ≤25% |
| Lu CL, 2016# | Taiwan | Observational. Follow-up: median 51.0 months (2–61.8 months) | 94 DM; 82 without DM | GA level was a strong predictor of the risk of death in patients with and without DM undergoing HD. The risk of mortality increased by 3.3% for each 1% rise in GA in all patients |
| Yajima T, 2016# | Japan | Observational. Follow-up: median 36.0 months | 78 DM2 | Serum albumin adjusted GA ≥21.2% was an independent predictor for mortality (HR: 3.76; 95% CI: 1.12–17.44; P=0.031) |
| Chen CW, 2017# | Germany | Retrospective study nested to a multicentre clinical trial. Follow up: mean 3.9 years | 1,053 DM | High GA levels in the baseline (fourth quartile GA >21%) had a 42% higher 4-year mortality compared to those in the first quartile (HR: 1.42; 95% CI: 1.09–1.85; P=0.009) |
| Hoshino J, 2018#** | Japan | Retrospective multicentre study. Follow up: 1 year | 22,441 DM | GA showed a linear association with 1-year mortality, with the lowest mortality at GA 15.6–18.2% |
| Abe M, 2019# | Japan | Retrospective multicentre study. Follow up: 2 years | 725 DM | GA ≥20.0% was significantly associated with a higher mortality in diabetic patients in peritoneal dialysis |
| Miyabe M, 2019# | Japan | Retrospective case-control. Follow up: 3 years | 44 DM in PD; 88 DM in HD | Higher GA levels (GA >18.0%) indicated significantly elevated risk for all-cause mortality |
| Hoshino J, 2020** | Japan | Retrospective multicentre study. Follow up: 3 years | 40,417 DM | In patients with GA ≥18% there was a linear association between GA levels and 3-year mortality |
#, studies included in the meta-analysis by Copur S, 2021; *, these studies evaluated the same cohort of patients; **, these studies evaluated the same cohort of patients in the first year of follow-up. GA, glycated albumin; DM2, diabetes mellitus type 2; DM, diabetes mellitus; PD, peritoneal dialysis; HD, haemodialysis; CVD, cardiovascular disease; HR, hazard ratio; CI, confidence interval; HbA1c, glycated haemoglobin; ESRD, end-stage renal disease.
Table 4 shows the main characteristics and findings of 15 studies reporting the association of GA and DRD, retinopathy or CVD in patients with diabetes without dialysis treatment (76-89).
Table 4
| Author, year publication | Study location | Type of study | Sample size (n) | Main findings |
|---|---|---|---|---|
| Ma WY, 2011 | Taiwan | Cross-sectional | 67 DM; 120 without DM | Increased GA concentrations were independently associated with renal dysfunction only in non-diabetic patients with CKD |
| Kondaveeti SB, 2013 | Indian | Case-control | 150 DM2 | The risk of microalbuminuria (high urinary albumin levels) increased with a poor glycaemic control measured by GA |
| Nathan DM, 2014 | USA | Case-control nested to multicentre cohort | 497 DM1 | Both HbA1c and GA were similarly associated with microvascular complications, but only HbA1c was associated with the cardiovascular complications |
| Selvin E, 2014 | USA | Prospective cohort. Follow up: 20 years | 958 DM; 11,348 without DM | GA was associated with a significantly increased risk of incident CKD. People without a history of diagnosed DM but with GA >15.2% (95th percentile) had raised risks of developing CKD (HR: 1.48; 95% CI: 1.20–1.83; P<0.001) compared with participants without DM and GA values below the 75th percentile. In people with DM the associations persisted statistically significant even after adjustments for traditional risk factors and for HbA1c and fasting glucose. Also, GA was strongly associated with prevalent retinopathy with OR >30.0 at high values of GA in patients with DM |
| Yoon HJ, 2015 | Korea | Retrospective longitudinal. Follow up: mean 2.8 years | 154 DM1 | GA levels were significantly associated with progression of DKD (OR: 2.03; 95% CI: 1.27–3.26; P=0.003) but not with CAA. HbA1c levels were not associated with either DKD or CAA |
| Jun JE, 2017 | Korea | Retrospective longitudinal. Follow up: 1 year | 449 DM2 | GA was significantly associated with higher risk of early DKD development, independently of HbA1c, and a better predictor of early DKD. Baseline and 1-year GA levels were stronger predictors of DKD development than baseline HbA1c levels |
| Park SB, 2017 | Korea | Retrospective longitudinal. Follow up: 33 months (12–46 months) | 369 DM2 | The variability in GA levels, indicated by the coefficient of variation of GA during the follow-up, was independently associated with the development and progression of DKD in patients with relatively well controlled DM2 (HbA1c <7.2%) but not in patients with relatively uncontrolled DM2 |
| Umayahara Y, 2017 | Japan | Cross-sectional | 613 DM2 | GA/HbA1c ratio was associated with diabetic retinopathy, but not with diabetic nephropathy in patients without overt proteinuria, reduced renal function or anaemia |
| Wang N, 2017 | China | Case-control | 206 DM2 | GA was associated with DKD (OR: 2.71; 95% CI: 1.15–4.01; P=0.019) but not HbA1c. Also, GA showed better performance for the prediction of DKD presence (AUC: 0.811; 95% CI: 0.752–0.869; P=0.005) than HbA1c (AUC: 0.580; 95% CI: 0.499–0.662; P=0.058). GA cut-off of 17.5% presented sensitivity of 0.761 and specificity of 0.644 for the diagnosis of DKD |
| Hong N 2018 | Korea | Retrospective cross-sectional | 204 DM1 | Elevated uNAG was associated with high GA/HbA1c ratio in patients with DM1 with early stage of DKD, independent of age and albuminuria |
| Huh JH, 2018 | Korea | Multicentre retrospective cross-sectional | 1,061 DM2 with normoalbuminuria and normal eGFR | GA was a good predictor of renal tubulopathy, indicated by uNAG abnormality, in patients with DM2 without overt DKD, regardless of HbA1c level or other conventional risk factors (AUC: 0.634; 95% CI: 0.646–0.899; P<0.001) |
| Raghav A, 2018 | India | Case-control | 355 DM2; 100 without DM | GA levels were more closely associated with the degree of DKD after stratification by CKD status than HbA1c levels |
| Vijayaraghavan B, 2020 | India | Cross-sectional | 194 DM | GA levels increased when the ejection fraction decreases and also it increases based on the number of vessels obstructed |
GA, glycated albumin; CVD, cardiovascular disease; DM, diabetes mellitus; DM1, diabetes mellitus type 1; DM2, diabetes mellitus type 2; eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease; HbA1c, glycated haemoglobin; HR, hazard ratio; CI, confidence interval; OR, odds ratio; CAA, carotid artery atherosclerosis; DKD, diabetes kidney disease; AUC, area under the curve; uNAG, urinary N-acetyl-β-D-glucosaminidase.
Two studies reported a positive association of GA with urinary N-acetyl-β-D-glucosaminidase (uNAG), an early marker of renal tubulopathy, in patients with type 1 (84) and type 2 (85) DM, with early DRD. Some longitudinal studies evaluated prospectively or retrospectively the association of GA with DRD and all showed that AG levels are associated with an increased risk of development and/or progression of DRD in type 1 and type 2 DM patients (79-82). Selvin et al. also showed that GA was strongly associated with prevalent retinopathy at high GA levels in patients with DM (79) and other study reported that the variability in GA levels rather than GA levels was associated with the development and progression of DRD (82). In general, observational studies reported the same relationship between GA and microvascular complications (54,58,76-78,83). In type 1 DM, both HbA1c and GA were similarly associated with microvascular complications (78). And in type 2 DM, studies showed that GA levels are associated with microalbuminuria, degree of renal dysfunction and in the prediction of DRD presence (54,58,76,77). However, Umayahara et al. reported that GA/HbA1c ratio was associated with diabetic retinopathy, but not with DRD (83). Very few studies evaluated the association of GA and CVD (78,80,86). GA levels were not associated with cardiovascular complications (78), and also were not associated with carotid artery atherosclerosis in type 1 DM patients (80). Although, Vijayaraghavan et al. reported that GA levels increase when the ejection fraction decreases and moreover it also increases based on the number of vessels obstructed (86).
Most of these studies were carried out in Asian countries (76,77,80-86). Only two studies were conducted by American centres (78,79).
Finally, Abe et al. (87) investigated the rate of ‘burnt-out diabetes’ condition in DM patients on peritoneal dialysis and reported that the rate was significantly decreased when taking the upper limit of GA values in the general population (16%) into account. Parrinello et al. (88) analysed the association of HbA1c, GA and other biomarkers with incident CVD, incident ESRD, and prevalent retinopathy in a large cohort of White and Blacks participants, with and without DM. They found that the prognostic value of GA, HbA1c and other biomarkers were similar by race with all DM long-term complications studied.
GA and post-transplantation diabetes mellitus (PTDM)
A specific type of DM that may occur after kidney transplantation is known as renal PTDM (1). Its development is associated with the use of immunosuppressive therapy, such as calcineurin inhibitors and corticosteroids. Only one study has specifically evaluated the accuracy of GA in the diagnosis of renal PTDM (90), but data is lacking about the performance of GA in the monitoring of DM in patients who underwent kidney transplantation. Estimated incidence of renal PTDM during the first year after transplant is about 20% (92). Studies have shown that the occurrence of PTDM increases the risk for CVD and mortality in kidney transplant recipients (93). The recommended test to detect this condition is oral glucose tolerance test (OGTT), once it has presented higher sensitivity to identify patients with PTDM during the first year after transplant when compared to FPG and HbA1c (1) Due to the inconvenience and high cost of performing an OGTT in all transplanted patients in the clinical setting, the use of FPG is highly spread. However, other diagnostic alternatives have also been accessed. The use of HbA1c alone in the first months after transplant has shown not to be adequate for the screening of PTDM, because it has presented low sensitivity and a great number of positive cases would be missed (94). In a cross-sectional study performed at the fourth month post-transplant (90), GA showed moderate diagnostic accuracy for renal PTDM when compared to OGTT and/or HbA1c as diagnostic criteria. The use of a single GA cut-point was not enough to properly screen and diagnose PTDM however GA ≥17% presented high specificity to rule in the disease. Nevertheless, this study did not show that GA was superior to HbA1c to detect PTDM in the initial months after kidney transplantation (90). There is still a gap in the literature regarding the association between GA levels and the development of adverse clinical outcomes in patients who develop PTDM after kidney transplantation or who had pre-existing DM.
Conclusions
GA, a short-term glycemic marker, has been pointed as an alternative test to HbA1C in patients with DM. It is indicated in clinical situations where HbA1c is not a reliable marker due to situations which may interference with the metabolism of hemoglobin. Also, it is especially indicated for patients on hemodialysis since its levels are not affected by the presence of anemia, uremia or hemolytic processes. In early 2000’s, a new enzymatic method to measure GA was described and showed to be simple, rapid, accurate and easily automatized. In the last years, many studies have evaluated the role of GA in the monitoring of DM. This review summarized the main findings of these studies.
Data from observational studies showed that the association of GA and HbA1c with measures of glycaemia is similar supporting that GA may be a substitute for HbA1c. Several studies showed that higher GA levels were associated with the risk of all-cause mortality in dialysis patients with DM regardless of the type of dialysis, whereas higher GA was not associated with cardiovascular mortality. These interactions varied from weak to modest in all studies. Other studies reported the association of GA and DRD, retinopathy or CVD in patients with diabetes without dialysis treatment. In patients with early nephropathy, several studies reported positive association between GA and microvascular complications although very few showed association of GA and DCV. The majority of these studies were carried out in Asian populations and the applicability of these results to all populations may not be straight forward. In addition, there is a lack of clinical trials and prospective studies that analysed GA in DM patients with and without CKD and these studies are warranted.
Although evidences show that GA may be a useful glycemic marker and prognostic factor in patients with CKD it should be used with caution in situations that its levels may be falsely altered as in the presence of massive proteinuria with low serum albumin. It should be highlighted that the choice of which test to use must be guided by the clinical patient features and accessibility to tests. Further, it is necessary an international consensus about laboratory issues and clinical use of GA, to guarantee its inclusion in the routine of clinical laboratory worldwide, thus improving the future controlling of DM patients.
In conclusion, GA is a promisor biomarker for the management of DM patients with and without CKD.
Acknowledgments
Funding: This work was partially supported by the Research Incentive Fund (FIPE) of the Hospital de Clinicas de Porto Alegre (HCPA) (FIPE/ HCPA, GPPG 190320); PhD scholarship from Programa de Excelência Acadêmica da Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES-PROEX) to FCC; and an undergraduate scholarship from Fundação de Amparo à Pesquisa do Rio Grande do Sul (FAPERGS) to LGS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Rafael Noal Moresco) for the series “Laboratory Medicine in Diabetic Kidney Disease” published in Journal of Laboratory and Precision Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the PRISMA Scoping Review reporting checklist (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-22-2/rc).
Peer Review File: Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-22-2/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-22-2/coif). The series “Laboratory Medicine in Diabetic Kidney Disease” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- American Diabetes Association. Standards of Medical Care in Diabetes—2021. Diabetes Care 2021;44:S1-232. [Crossref] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int 2020;98:S1-S115. [Crossref]
- Sacks DB, Arnold M, Bakris GL, et al. Executive summary: guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem 2011;57:793-8. [Crossref] [PubMed]
- Cavagnolli G, Pimentel AL, Freitas PA, et al. Factors affecting A1C in non-diabetic individuals: Review and meta-analysis. Clin Chim Acta 2015;445:107-14. [Crossref] [PubMed]
- Cavagnolli G, Pimentel AL, Freitas PA, et al. Effect of ethnicity on HbA1c levels in individuals without diabetes: Systematic review and meta-analysis. PLoS One 2017;12:e0171315. [Crossref] [PubMed]
- Freitas PAC, Ehlert LR, Camargo JL. Glycated albumin: a potential biomarker in diabetes. Arch Endocrinol Metab 2017;61:296-304. [Crossref] [PubMed]
- Gan T, Liu X, Xu G. Glycated Albumin Versus HbA1c in the Evaluation of Glycemic Control in Patients With Diabetes and CKD. Kidney Int Rep 2017;3:542-54. [Crossref] [PubMed]
- Zendjabil M. Glycated albumin. Clin Chim Acta 2020;502:240-4. [Crossref] [PubMed]
- Dozio E, Di Gaetano N, Findeisen P, et al. Glycated albumin: from biochemistry and laboratory medicine to clinical practice. Endocrine 2017;55:682-90. [Crossref] [PubMed]
- Silva JF, Pimentel AL, Camargo JL. Effect of iron deficiency anaemia on HbA1c levels is dependent on the degree of anaemia. Clin Biochem 2016;49:117-20. [Crossref] [PubMed]
- Kouzuma T, Usami T, Yamakoshi M, et al. An enzymatic method for the measurement of glycated albumin in biological samples. Clin Chim Acta 2002;324:61-71. [Crossref] [PubMed]
- Paroni R, Ceriotti F, Galanello R, et al. Performance characteristics and clinical utility of an enzymatic method for the measurement of glycated albumin in plasma. Clin Biochem 2007;40:1398-405. [Crossref] [PubMed]
- Kohzuma T, Koga M. Lucica GA-L glycated albumin assay kit: a new diagnostic test for diabetes mellitus. Mol Diagn Ther 2010;14:49-51. [Crossref] [PubMed]
- Paleari R, Bonetti G, Callà C, et al. Multicenter evaluation of an enzymatic method for glycated albumin. Clin Chim Acta 2017;469:81-6. [Crossref] [PubMed]
- Freitas PAC, Ehlert LR, Camargo JL. Comparison between two enzymatic methods for glycated albumin. Anal Methods 2016;8:8173-8. [Crossref]
- Testa R, Ceriotti F, Guerra E, et al. Glycated albumin: correlation to HbA1c and preliminary reference interval evaluation. Clin Chem Lab Med 2017;55:e31-3. [Crossref] [PubMed]
- Ferrario L, Schettini F, Avogaro A, et al. Glycated Albumin for Glycemic Control in T2DM Population: A Multi-Dimensional Evaluation. Clinicoecon Outcomes Res 2021;13:453-64. [Crossref] [PubMed]
- Freitas PAC, Hernandez MK, Camargo JL. Factors associated with glycated albumin in adults without diabetes. Med Pharm Rep 2021;94:170-5. [PubMed]
- Liang L, He H, Zeng Y, et al. Evaluation of biological variation of glycated hemoglobin and glycated albumin in healthy Chinese subjects. J Clin Lab Anal 2019;33:e22715. [Crossref] [PubMed]
- Okada T, Nakao T, Matsumoto H, et al. Influence of proteinuria on glycated albumin values in diabetic patients with chronic kidney disease. Intern Med 2011;50:23-9. [Crossref] [PubMed]
- Ueda S, Nagai K, Yokota N, et al. Influence of albumin leakage on glycated albumin in patients with type 2 diabetes undergoing hemodialysis. J Artif Organs 2019;22:264-7. [Crossref] [PubMed]
- Miyawaki J, Okuno S, Mori K, et al. Inverse association of fat mass, but not lean mass, with glycated albumin in hemodialysis patients with or without diabetes mellitus. Ren Fail 2019;41:808-13. [Crossref] [PubMed]
- Okada T, Nakao T, Matsumoto H, et al. Influence of age and nutritional status on glycated albumin values in hemodialysis patients. Intern Med 2009;48:1495-9. [Crossref] [PubMed]
- Lee MJ, Kwon YE, Park KS, et al. Glycemic Control Modifies Difference in Mortality Risk Between Hemodialysis and Peritoneal Dialysis in Incident Dialysis Patients With Diabetes: Results From a Nationwide Prospective Cohort in Korea. Medicine (Baltimore) 2016;95:e3118. [Crossref] [PubMed]
- Wu MS, Yu CC, Yang CW, et al. Poor pre-dialysis glycaemic control is a predictor of mortality in type II diabetic patients on maintenance haemodialysis. Nephrol Dial Transplant 1997;12:2105-10. [Crossref] [PubMed]
- Li X, Xu X, Liu J, et al. HbA1c and survival in maintenance hemodialysis patients with diabetes in Han Chinese population. Int Urol Nephrol 2014;46:2207-14. [Crossref] [PubMed]
- Park JI, Bae E, Kim YL, et al. Glycemic Control and Mortality in Diabetic Patients Undergoing Dialysis Focusing on the Effects of Age and Dialysis Type: A Prospective Cohort Study in Korea. PLoS One 2015;10:e0136085. [Crossref] [PubMed]
- Wu MS, Yu CC, Wu CH, et al. Pre-dialysis glycemic control is an independent predictor of mortality in type II diabetic patients on continuous ambulatory peritoneal dialysis. Perit Dial Int 1999;19:S179-83. [Crossref] [PubMed]
- Yu CC, Wu MS, Wu CH, et al. Predialysis glycemic control is an independent predictor of clinical outcome in type II diabetics on continuous ambulatory peritoneal dialysis. Perit Dial Int 1997;17:262-8. [Crossref] [PubMed]
- de Boer IHDCCT/EDIC Research Group. Kidney disease and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care 2014;37:24-30. [Crossref] [PubMed]
- DCCT/EDIC research group. Effect of intensive diabetes treatment on albuminuria in type 1 diabetes: long-term follow-up of the Diabetes Control and Complications Trial and Epidemiology of Diabetes Interventions and Complications study. Lancet Diabetes Endocrinol 2014;2:793-800. [Crossref] [PubMed]
- DCCT/EDIC Research Group. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med 2011;365:2366-76. [Crossref] [PubMed]
- Zoungas S, Arima H, Gerstein HC, et al. Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta-analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol 2017;5:431-7. [Crossref] [PubMed]
- Zoungas S, Chalmers J, Ninomiya T, et al. Association of HbA1c levels with vascular complications and death in patients with type 2 diabetes: evidence of glycaemic thresholds. Diabetologia 2012;55:636-43. [Crossref] [PubMed]
- Chujo K, Shima K, Tada H, et al. Indicators for blood glucose control in diabetics with end-stage chronic renal disease: GHb vs. glycated albumin (GA). J Med Invest 2006;53:223-8. [Crossref] [PubMed]
- Inaba M, Okuno S, Kumeda Y, et al. Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: effect of anemia and erythropoietin injection. J Am Soc Nephrol 2007;18:896-903. [Crossref] [PubMed]
- Nagayama H, Inaba M, Okabe R, et al. Glycated albumin as an improved indicator of glycemic control in hemodialysis patients with type 2 diabetes based on fasting plasma glucose and oral glucose tolerance test. Biomed Pharmacother 2009;63:236-40. [Crossref] [PubMed]
- Uzu T, Hatta T, Deji N, et al. Target for glycemic control in type 2 diabetic patients on hemodialysis: effects of anemia and erythropoietin injection on hemoglobin A(1c). Ther Apher Dial 2009;13:89-94. [Crossref] [PubMed]
- Freedman BI, Shenoy RN, Planer JA, et al. Comparison of glycated albumin and hemoglobin A1c concentrations in diabetic subjects on peritoneal and hemodialysis. Perit Dial Int 2010;30:72-9. [Crossref] [PubMed]
- Tajiri Y, Sato S, Hattori S, et al. Validity of haemoglobin A1c and glycoalbumin for an appropriate evaluation of glycaemic control in Japanese diabetic patients with chronic renal failure. NDT Plus 2010;3:507-9. [PubMed]
- Kim JK, Park JT, Oh HJ, et al. Estimating average glucose levels from glycated albumin in patients with end-stage renal disease. Yonsei Med J 2012;53:578-86. [Crossref] [PubMed]
- Vos FE, Schollum JB, Coulter CV, et al. Assessment of markers of glycaemic control in diabetic patients with chronic kidney disease using continuous glucose monitoring. Nephrology (Carlton) 2012;17:182-8. [Crossref] [PubMed]
- Chen FK, Sun XF, Zhang D, et al. Glycated albumin may be a choice, but not an alternative marker of glycated hemoglobin for glycemic control assessment in diabetic patients undergoing maintenance hemodialysis. Chin Med J (Engl) 2013;126:3295-300. [PubMed]
- Konya J, Ng JM, Cox H, et al. Use of complementary markers in assessing glycaemic control in people with diabetic kidney disease undergoing iron or erythropoietin treatment. Diabet Med 2013;30:1250-4. [Crossref] [PubMed]
- Lee SY, Chen YC, Tsai IC, et al. Glycosylated hemoglobin and albumin-corrected fructosamine are good indicators for glycemic control in peritoneal dialysis patients. PLoS One 2013;8:e57762. [Crossref] [PubMed]
- Sany D, Elshahawy Y, Anwar W. Glycated albumin versus glycated hemoglobin as glycemic indicator in hemodialysis patients with diabetes mellitus: variables that influence. Saudi J Kidney Dis Transpl 2013;24:260-73. [Crossref] [PubMed]
- Harada K, Sumida K, Yamaguchi Y, et al. Relationship between the accuracy of glycemic markers and the chronic kidney disease stage in patients with type 2 diabetes mellitus. Clin Nephrol 2014;82:107-14. [Crossref] [PubMed]
- Fukami K, Shibata R, Nakayama H, et al. Serum albumin-adjusted glycated albumin reflects glycemic excursion in diabetic patients with severe chronic kidney disease not treated with dialysis. J Diabetes Complications 2015;29:913-7. [Crossref] [PubMed]
- Kim IY, Kim MJ, Lee DW, et al. Glycated albumin is a more accurate glycaemic indicator than haemoglobin A1c in diabetic patients with pre-dialysis chronic kidney disease. Nephrology (Carlton) 2015;20:715-20. [Crossref] [PubMed]
- Williams ME, Mittman N, Ma L, et al. The Glycemic Indices in Dialysis Evaluation (GIDE) study: Comparative measures of glycemic control in diabetic dialysis patients. Hemodial Int 2015;19:562-71. [Crossref] [PubMed]
- Hayashi A, Takano K, Masaki T, et al. Distinct biomarker roles for HbA1c and glycated albumin in patients with type 2 diabetes on hemodialysis. J Diabetes Complications 2016;30:1494-9. [Crossref] [PubMed]
- Kobayashi H, Abe M, Yoshida Y, et al. Glycated Albumin versus Glycated Hemoglobin as a Glycemic Indicator in Diabetic Patients on Peritoneal Dialysis. Int J Mol Sci 2016;17:619. [Crossref] [PubMed]
- Tsuruta Y, Ichikawa A, Kikuchi K, et al. Glycated albumin is a better indicator of the glucose excursion than predialysis glucose and hemoglobin A1c in hemodialysis patients. Renal Replacement Therapy 2016;2:1-5. [Crossref]
- Wang N, Xu Z, Han P, et al. Glycated albumin and ratio of glycated albumin to glycated hemoglobin are good indicators of diabetic nephropathy in type 2 diabetes mellitus. Diabetes Metab Res Rev 2017; [Crossref] [PubMed]
- Yajima T, Yajima K, Hayashi M, et al. Serum albumin-adjusted glycated albumin as a better indicator of glycemic control in Type 2 diabetes mellitus patients with short duration of hemodialysis. Diabetes Res Clin Pract 2017;130:148-53. [Crossref] [PubMed]
- Divani M, Georgianos PI, Didangelos T, et al. Comparison of Glycemic Markers in Chronic Hemodialysis Using Continuous Glucose Monitoring. Am J Nephrol 2018;47:21-9. [Crossref] [PubMed]
- Jung M, Warren B, Grams M, et al. Performance of non-traditional hyperglycemia biomarkers by chronic kidney disease status in older adults with diabetes: Results from the Atherosclerosis Risk in Communities Study. J Diabetes 2018;10:276-85. [Crossref] [PubMed]
- Raghav A, Ahmad J, Noor S, et al. Glycated albumin and the risk of chronic kidney disease in subjects with Type 2 Diabetes: A study in North Indian Population. Diabetes Metab Syndr 2018;12:381-5. [Crossref] [PubMed]
- Bellia C, Cosma C, Lo Sasso B, et al. Glycated albumin as a glycaemic marker in patients with advanced chronic kidney disease and anaemia: a preliminary report. Scand J Clin Lab Invest 2019;79:293-7. [Crossref] [PubMed]
- Zelnick LR, Batacchi ZO, Ahmad I, et al. Continuous Glucose Monitoring and Use of Alternative Markers To Assess Glycemia in Chronic Kidney Disease. Diabetes Care 2020;43:2379-87. [Crossref] [PubMed]
- Okada T, Nakao T, Matsumoto H, et al. Association between markers of glycemic control, cardiovascular complications and survival in type 2 diabetic patients with end-stage renal disease. Intern Med 2007;46:807-14. [Crossref] [PubMed]
- Fukuoka K, Nakao K, Morimoto H, et al. Glycated albumin levels predict long-term survival in diabetic patients undergoing haemodialysis. Nephrology (Carlton) 2008;13:278-83. [Crossref] [PubMed]
- Kumeda Y, Inaba M, Shoji S, et al. Significant correlation of glycated albumin, but not glycated haemoglobin, with arterial stiffening in haemodialysis patients with type 2 diabetes. Clin Endocrinol (Oxf) 2008;69:556-61. [Crossref] [PubMed]
- Yamada S, Inaba M, Shidara K, et al. Association of glycated albumin, but not glycated hemoglobin, with peripheral vascular calcification in hemodialysis patients with type 2 diabetes. Life Sci 2008;83:516-9. [Crossref] [PubMed]
- Freedman BI, Andries L, Shihabi ZK, et al. Glycated albumin and risk of death and hospitalizations in diabetic dialysis patients. Clin J Am Soc Nephrol 2011;6:1635-43. [Crossref] [PubMed]
- Murea M, Moran T, Russell GB, et al. Glycated albumin, not hemoglobin A1c, predicts cardiovascular hospitalization and length of stay in diabetic patients on dialysis. Am J Nephrol 2012;36:488-96. [Crossref] [PubMed]
- Shafi T, Sozio SM, Plantinga LC, et al. Serum fructosamine and glycated albumin and risk of mortality and clinical outcomes in hemodialysis patients. Diabetes Care 2013;36:1522-33. [Crossref] [PubMed]
- Isshiki K, Nishio T, Isono M, et al. Glycated albumin predicts the risk of mortality in type 2 diabetic patients on hemodialysis: evaluation of a target level for improving survival. Ther Apher Dial 2014;18:434-42. [Crossref] [PubMed]
- Lu CL, Ma WY, Lin YF, et al. Glycated Albumin Predicts Long-term Survival in Patients Undergoing Hemodialysis. Int J Med Sci 2016;13:395-402. [Crossref] [PubMed]
- Yajima T, Yajima K, Hayashi M, et al. Serum albumin-adjusted glycated albumin is a better predictor of mortality in diabetic patients with end-stage renal disease on hemodialysis. J Diabetes Complications 2016;30:786-9. [Crossref] [PubMed]
- Chen CW, Drechsler C, Suntharalingam P, et al. High Glycated Albumin and Mortality in Persons with Diabetes Mellitus on Hemodialysis. Clin Chem 2017;63:477-85. [Crossref] [PubMed]
- Hoshino J, Hamano T, Abe M, et al. Glycated albumin versus hemoglobin A1c and mortality in diabetic hemodialysis patients: a cohort study. Nephrol Dial Transplant 2018;33:1150-8. [Crossref] [PubMed]
- Abe M, Hamano T, Hoshino J, et al. Glycemic control and survival in peritoneal dialysis patients with diabetes: A 2-year nationwide cohort study. Sci Rep 2019;9:3320. [Crossref] [PubMed]
- Miyabe M, Kurajoh M, Mori K, et al. Superiority of glycated albumin over glycated haemoglobin as indicator of glycaemic control and predictor of all-cause mortality in patients with type 2 diabetes mellitus receiving peritoneal dialysis. Ann Clin Biochem 2019;56:684-91. [Crossref] [PubMed]
- Hoshino J, Abe M, Hamano T, et al. Glycated albumin and hemoglobin A1c levels and cause-specific mortality by patients' conditions among hemodialysis patients with diabetes: a 3-year nationwide cohort study. BMJ Open Diabetes Res Care 2020;8:e001642. [Crossref] [PubMed]
- Ma WY, Wu CC, Pei D, et al. Glycated albumin is independently associated with estimated glomerular filtration rate in nondiabetic patients with chronic kidney disease. Clin Chim Acta 2011;412:583-6. [Crossref] [PubMed]
- Kondaveeti SB. Evaluation of glycated albumin and microalbuminuria as early risk markers of nephropathy in type 2 diabetes mellitus. J Clin Diagn Res 2013;7:1280-3. [PubMed]
- Nathan DM, McGee P, Steffes MW, et al. Relationship of glycated albumin to blood glucose and HbA1c values and to retinopathy, nephropathy, and cardiovascular outcomes in the DCCT/EDIC study. Diabetes 2014;63:282-90. [Crossref] [PubMed]
- Selvin E, Rawlings AM, Grams M, et al. Fructosamine and glycated albumin for risk stratification and prediction of incident diabetes and microvascular complications: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol 2014;2:279-88. [Crossref] [PubMed]
- Yoon HJ, Lee YH, Kim SR, et al. Glycated albumin and the risk of micro- and macrovascular complications in subjects with type 1 diabetes. Cardiovasc Diabetol 2015;14:53. [Crossref] [PubMed]
- Jun JE, Hur KY, Lee YB, et al. Glycated albumin predicts the development of early diabetic nephropathy in patients with type 2 diabetes. Diabetes Metab 2018;44:178-80. [Crossref] [PubMed]
- Park SB, Kim SS, Kim IJ, et al. Variability in glycated albumin levels predicts the progression of diabetic nephropathy. J Diabetes Complications 2017;31:1041-6. [Crossref] [PubMed]
- Umayahara Y, Fujita Y, Watanabe H, et al. Association of glycated albumin to HbA1c ratio with diabetic retinopathy but not diabetic nephropathy in patients with type 2 diabetes. Clin Biochem 2017;50:270-3. [Crossref] [PubMed]
- Hong N, Lee M, Park S, et al. Elevated urinary N-acetyl-β-D-glucosaminidase is associated with high glycoalbumin-to-hemoglobin A1c ratio in type 1 diabetes patients with early diabetic kidney disease. Sci Rep 2018;8:6710. [Crossref] [PubMed]
- Huh JH, Lee M, Park SY, et al. Glycated Albumin Is a More Useful Glycation Index than HbA1c for Reflecting Renal Tubulopathy in Subjects with Early Diabetic Kidney Disease. Diabetes Metab J 2018;42:215-23. [Crossref] [PubMed]
- Vijayaraghavan B, Padmanabhan G, Ramanathan K. Determination of serum glycated albumin and high sensitivity C - reactive protein in the insight of cardiovascular complications in diabetic chronic kidney disease patients. Afr Health Sci 2020;20:308-13. [Crossref] [PubMed]
- Abe M, Hamano T, Hoshino J, et al. Rate of the "burnt-out diabetes" phenomenon in patients on peritoneal dialysis. Diabetes Res Clin Pract 2018;143:254-62. [Crossref] [PubMed]
- Parrinello CM, Sharrett AR, Maruthur NM, et al. Racial Differences in and Prognostic Value of Biomarkers of Hyperglycemia. Diabetes Care 2016;39:589-95. [Crossref] [PubMed]
- Fukami K, Shibata R, Nakayama H, et al. Serum albumin-adjusted glycated albumin is a better indicator of glycaemic control in diabetic patients with end-stage renal disease not on haemodialysis. Ann Clin Biochem 2015;52:488-96. [Crossref] [PubMed]
- Pimentel AL, Hernandez MK, Freitas PAC, et al. The usefulness of glycated albumin for post-transplantation diabetes mellitus after kidney transplantation: A diagnostic accuracy study. Clin Chim Acta 2020;510:330-6. [Crossref] [PubMed]
- Copur S, Siriopol D, Afsar B, et al. Serum glycated albumin predicts all-cause mortality in dialysis patients with diabetes mellitus: meta-analysis and systematic review of a predictive biomarker. Acta Diabetol 2021;58:81-91. [Crossref] [PubMed]
- Sharif A, Hecking M, de Vries AP, et al. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: recommendations and future directions. Am J Transplant 2014;14:1992-2000.
- Eide IA, Halden TAS, Hartmann A, et al. Associations Between Posttransplantation Diabetes Mellitus and Renal Graft Survival. Transplantation 2017;101:1282-9. [Crossref] [PubMed]
- Pimentel AL, Cavagnolli G, Camargo JL. Diagnostic accuracy of glycated hemoglobin for post-transplantation diabetes mellitus after kidney transplantation: systematic review and meta-analysis. Nephrol Dial Transplant 2017;32:565-72. [Crossref] [PubMed]
Cite this article as: Chume FC, Schiavenin LG, Freitas PAC, Pimentel AL, Camargo JL. The usefulness of glycated albumin in patients with diabetes and renal disease: a scoping review. J Lab Precis Med 2022;7:12.


