Investigative algorithms for disorders affecting plasma magnesium: a narrative review
Introduction
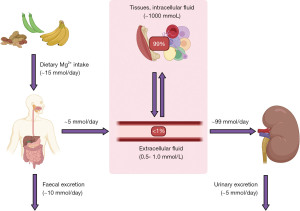
Magnesium is an abundant intracellular cation and is an essential cofactor for multiple enzymes. Most importantly, magnesium helps in the transfer of inorganic phosphate in many metabolic reactions such as in glycolysis, gluconeogenesis, citric acid cycle and ATP synthesis (1). Only 1% of total body magnesium resides in the extracellular space, 50–65% is complexed in bone and acts as a buffer pool to maintain magnesium homeostasis, and the remainder is found in the intracellular space (Figure 1) (2). Of the total extracellular magnesium, just over half circulates as a free divalent cation, 30% is bound to plasma proteins (particularly albumin), and 15% is complexed with anions (carbonate, phosphate, citrates, hydroxide and chloride). Therefore, changes in protein binding or anion quantity will influence serum magnesium concentration if measuring total magnesium (the most commonly used analytical method to quantitate magnesium in the UK) (1,3). Serum magnesium has a concentration of 0.7–1.0 mmol/L, although this will be method and population dependent, and is not always a reliable indicator of the status of total body magnesium (3). The following algorithms aim to provide a rough guide on using laboratory diagnostics to evaluate the causes of magnesium disturbances. We present the following article in accordance with the Narrative Review reporting checklist (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-22-6/rc).
Methods
The narrative literature review was undertaken with review of Medline, Google Scholar, OMIM and seminal texts. The search was performed from September 2021 to January 2022 from database inception. Language was restricted to English. Diagnostic algorithms were created from synthesis of the information obtained from literature review. More information is found in the supplementary information (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search (specified to date, month and year) | September 2021 to January 2022 |
| Databases and other sources searched | Medline, Google Scholar, OMIM |
| Search terms used (including MeSH and free text search terms and filters). Examples include: | Hypermagnesaemia (hypermagenesemia), Hypomagnesaemia (hypomagnesemia), Magnesium, Diagnosis, Investigation, Causes, Aetiology, Paediatrics, Pregnancy, Human |
| Timeframe | From database inception to January 2022 |
| Inclusion and exclusion criteria (study type, language restrictions, etc.) | All papers and reviews were included restricted to English |
| Selection process (who conducted the selection, whether it was conducted independently, how consensus was obtained, etc.) | C Darragh-Hickey, S Kaur and KE. Shipman conducted initial search, with refinement by all other authors to obtain consensus and agreement |
| Any additional considerations, if applicable | Seminal texts were also searched and the references of important articles and texts were obtained and checked for relevance |
Metabolism and regulation
Magnesium is found in a variety of foodstuffs including green vegetables, legumes, whole grains, nuts, seeds, rice and fruits. Bodily magnesium levels are regulated by intestinal absorption (mainly in the colon and distal small intestine), hormonal control of stores in the bone, and renal reabsorption, specifically at the ascending limb of the loop of Henle (Figure 1). Only 30–50% of dietary magnesium is normally absorbed by the gastrointestinal tract, increasing to around 80% in hypomagnesaemia (2). Magnesium is absorbed in the gastrointestinal tract using luminal membrane transporters, namely Transient Receptor Potential Melastatin type 6 and 7 (TRPM-6 and -7), and paracellularly as tight junction claudins are limited (Figure 2) (2). TRPM-6 and -7 have close homology. TRPM-7 is ubiquitously expressed whereas TRPM-6 is mainly localised to the apical surface of the colon and renal epithelia. Following TRPM absorption, magnesium enters the bloodstream through the solute carrier family 41 member 1 (SLC41A1), a basolateral membrane sodium-magnesium exchanger (2,4).
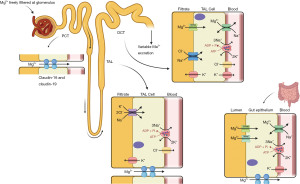
The kidneys reabsorb 90–95% of filtered magnesium—10–20% in the proximal convoluted tubule (PCT), 50–70% in the thick ascending loop of Henle (TAL) and 5–10% in the distal convoluted tubule (DCT) (2,5). Paracellular absorption occurs in the PCT and TAL, and fine-tuning transcellular absorption in the DCT using TRPM-6 and SLC41A1 channels as described above (Figure 2) (4).
Hypomagnesaemia and hypocalcaemia stimulate parathyroid hormone (PTH) secretion, which in turn regulates calcium homeostasis. PTH facilitates bone resorption which can cause release of calcium and magnesium ions into the circulation. PTH also causes vitamin D activation which causes renal reabsorption of both calcium and magnesium (1). This therefore means that hypermagnesaemia and hypercalcaemia can coincide in hyperparathyroidism. In the kidney, both the PTH receptor type 1 and the calcium-sensing receptor (CASR) activate the Na-K-Cl cotransporter (NKCC2) in the TAL promoting reabsorption of magnesium (6). Outside of hyperparathyroidism, hypercalcaemia can cause hypomagnesaemia by competing for protein binding in the circulation and for renal uptake resulting in hypermagnesuria.
Importantly, total magnesium assays may be misleading. Adjustment formulae which use albumin concentration exist, but unlike calcium, they have been shown to have little clinical relevance (3,7). Globally, ionised magnesium assays are in use, and it is worth understanding if the reported value is adjusted for pH and calcium concentration. Such adjustments may not be valid at extremes of pH for example, and the adjusted value may also not represent the true in vivo status. Therefore, no single test is easily considered gold standard. Theoretically, ionised magnesium may be the most likely candidate. However, there is a lack of evidence on what the reference range should be, how to treat abnormalities and what a treatment target should be, what the relationship is with total magnesium (which will remain complicated by the variables that affect ionised magnesium such as calcium concentration, pH, temperature and dilution), and if treatment based on ionised magnesium targets will make a clinically significant improvement to patient outcomes (8). More research is required before we can be more confident on which test to use and how this relates to management protocols.
Hypomagnesaemia
Due to large magnesium stores in bone, it can take months/years for magnesium deficiency to manifest biochemically and clinically. To complicate the diagnosis, it is possible to have a total body deficiency but with a normal serum total magnesium concentration (9). A magnesium loading test can be useful to confirm deficiency in patients with normomagnesaemia and normal renal function. The test involves administration of magnesium as sulphate or chloride intravenously and monitoring the urinary magnesium excretion over 24 h. Patients with latent magnesium deficiency tend to retain more and excrete less of the load than those who are magnesium replete. However, the magnesium loading test is usually reserved for cases where there is a strong clinical suspicion of magnesium deficiency, despite normal serum/plasma magnesium concentration. The loading test is not routinely performed due to its many contraindications and the uncertainty around its clinical usefulness (10-12).
On the other hand, a low serum magnesium concentration can be misleading if there are substantial changes to protein levels or acid-base status (3,10). This infers that some low serum magnesium concentrations may not be clinically relevant or indicative of total body magnesium deficiency, since serum magnesium concentration can drop quickly and stores are usually slow to respond. In these cases, total body magnesium is replete and supplementing magnesium will not benefit the patient (3).
Once hypomagnesaemia is confirmed, we present an algorithm from a laboratory investigatory approach to diagnosis (Figure 3). Initially the focus of the algorithm is on establishing if the hypomagnesaemia is real via consideration of albumin adjustment, albumin concentration also being a useful marker of acute severe illness, and calcium concentration as a marker of possible contamination of the specimen by cation chelators found in types of specimen tubes as well as intracellular shift. Low calcium due to cation chelators can be subtle. However, in moderate contamination, the calcium concentration will be unexpectedly low, e.g., no evidence of disease in history or bloods and may be out of keeping with normal physiology, e.g., normal renal function and potassium concentration >10 mmol/L and calcium concentration <1 mmol/L in ethylenediaminetetraacetic acid (EDTA contamination) (13). If earlier results are available, then rapid shifts in selected analytes can be indicative. Citrate, EDTA and oxalate are common tube additives that chelate cations; primarily designed to chelate calcium to inhibit clotting to produce plasma specimens. Refeeding syndrome, hungry bone disease and treatment of diabetic ketoacidosis can cause magnesium redistribution to the intracellular space and should be evident from history and clinical assessment (multiple biochemical abnormalities, e.g., acidosis, hypocalcaemia, hypokalaemia as well as administration of medications and past medical history).
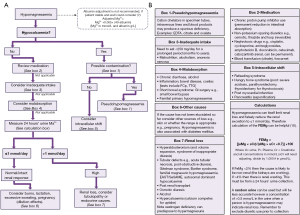
Having established the hypomagnesaemia is real and not due to acute illness or changes in binding or tissue compartment, then risk factors for deficiency should be sought in the history. Medication, dietary intake and history of gastrointestinal disorders therefore form the next steps of establishing the cause. Gastrointestinal diseases (e.g., inflammatory bowel disease), surgery (e.g., colectomy) or chronic alcoholism can also predispose individuals to hypomagnesaemia (1,14). At this point, assessment for possible renal wasting is recommended with a 24-h urine collection for magnesium concentration. Chronic hypomagnesaemia can occur in the elderly due to a combination of reduced dietary intake and urinary magnesium wasting (1).
Establishing if renal loss is the cause of hypomagnesaemia can also be complicated by the fact that magnesium concentration affects renal reabsorption. For example, if magnesium is low due to renal loss, the deficiency itself reduces the amount of magnesium excreted, which may mean the urine magnesium concentration is spuriously low appearing to rule out hypermagnesuria (15). Conversely, if a magnesium infusion has been given, the high plasma magnesium concentration will stimulate renal magnesium wasting with an estimated 50% of an intravenous magnesium load being lost in the urine (10). Therefore, although a 24-h urine magnesium collection combined with fractional excretion calculation may be the most useful tests to estimate renal loss, the results can be unreliable due to treatments administered and the low magnesium itself (10).
Primary renal disease can lead to renal magnesium wasting, e.g., tubulopathy. Tubulopathies can be evident by loss of other electrolytes, e.g., potassium and phosphate, and presence of protein and glucose in the urine. Chronic kidney disease patients though often have hypomagnesaemia as a result of a reduced vitamin 1,25(OH)2D3 concentration, which causes renal losses as magnesium reabsorption is stimulated by 1,25(OH)2D3 (2). Persistently low eGFR and high parathyroid hormone concentration can indicate a chronic kidney condition as can hyperkalaemia, acidosis, and hyperuricemia. Magnesium is a cofactor for 25-hydroxylase and 1-α-hydroxylase; hence, hypomagnesaemia can exacerbate its own deficiency and cause a magnesium dependent vitamin D resistant rickets (1). Similarly, as previously mentioned, magnesium is needed for PTH secretion. Therefore, extremely low concentrations of magnesium results in PTH suppression, which will also exacerbate the deficiency (1). Renal wasting of magnesium can therefore indicate either primary renal disease, endocrine disorders as mentioned above and hyperaldosteronism, drugs, e.g., alcohol or competition from calcium in hypercalcaemia.
If renal wasting is not identified, noting the difficulties with interpretation of urine magnesium concentration, then consider loss from skin, e.g., burns, breast milk during lactation or dilutional effects such as in pregnancy. Multiple other related electrolyte (hypokalaemia, hypocalcaemia, hypophosphataemia) and acid base disorders (respiratory/metabolic alkalosis or mixed disorders) can occur with hypomagnesaemia, depending on the underlying cause. Please refer to companion articles in the series to aid with diagnosis if another abnormality is particularly marked. Note disorders of potassium do not primarily cause magnesium disorders but commonly coexist, i.e., causes of gastrointestinal and renal losses or tissue translocation will affect both analytes (and calcium). However, magnesium concentration does directly control potassium concentration by affecting the function of potassium pumps, channels and membrane charge and permeability (16).
Hypermagnesaemia
Hypermagnesaemia is significantly rarer than hypomagnesaemia (17). A diagnostic algorithm is presented to aid determination of the aetiology (Figure 4). Hypermagnesaemia is primarily due to ingestion of magnesium containing drugs, e.g., laxatives such as Epsom salts, combined with unregulated absorption due to gastrointestinal pathology, or lack of excretion from renal failure (18-20). Increased intake via intravenous infusion, ingestion of magnesium rich sea water and milk alkali syndrome, can all rarely lead to hypermagnesaemia. Therefore, drug and past medical history form an essential start to the diagnostic approach. Note lithium therapy increases the renal reabsorption of magnesium, as does hypothyroidism [defined by low thyroxine (T4) and triiodothyronine (T3)], adrenal insufficiency (hypocortisolaemia particularly after stimulation with synthetic adrenocorticotropic hormone which can be associated with postural hypotension, skin pigmentation, and hyponatraemia with hyperkalaemia) and hyperparathyroidism (hypercalcaemia with normal or high PTH concentration). It is worth noting that hypercalcaemia can cause hypomagnesaemia due to PTH suppression, but hypercalaemia due to hyperparathyroidism (or the very rare familial hypocalciuric hypercalcaemia which presents like primary hyperparathyroidism except there is no history or normocalcaemia on previous blood tests and calcium:creatinine clearance ratio is <0.01) is associated with hypermagnesaemia (21).
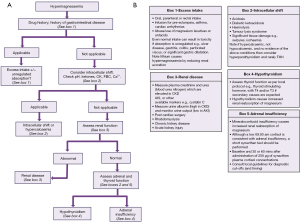
To approach the diagnosis therefore the next step, after excluding a likely cause from the history, is to focus on the results of laboratory investigations. Initially, intracellular shifts caused by acidosis or tissue lysis and hypercalcaemia should be ruled out, followed by primary renal disease (renal failure potentially also contributing to the hypermagnesaemia associated with acidosis and tissue lysis syndromes). Tissue lysis conditions will also be associated with other blood test abnormalities including raised creatinine kinase, aspartate aminotransferase and potassium for example. Finally endocrine causes such as adrenal insufficiency and hypothyroidism should be ruled out. The order being dictated by order of patient clerking, those most obvious on clinical assessment (for example tumour lysis or rhabdomyolysis should be detected in the history) and clinical severity.
Pregnancy and paediatrics
Pregnancy is associated with multiple metabolic changes; perhaps most notable is significant haemodilution. Magnesium concentration is significantly affected, with approximately a 0.1 mmol/L reduction at both lower and upper ends of the reference interval. However, this is most marked at the lower limit (up to 0.4 mmol/L lower, i.e., a 30% reduction) at 34–38 weeks gestation (22-24). Interestingly, ionised and intracellular magnesium concentrations also fall in pregnancy, and urine excretion increases by 25% (19). A small study suggested that there might be benefit in magnesium supplementation during pregnancy, but it was underpowered for the number of comparisons (25). It is also worth noting that oestrogen acts to stimulate TRPM6 expression. Therefore, menopause may be a risk factor hypermagnesuria, which in turn can induce hypomagnesaemia (2,26).
The upper limit of the reference interval for magnesium is higher in children, particularly in the first 15 days of life, but more subtly until 1 year of age where the range equates well to adult ranges. However, this could represent the influence of venesection technique and haematocrit (27). Breast milk contains between 15–64 mg/L of magnesium, and infants must supplement their micronutrients to satisfy their needs while growing. Formula powder has 42 mg/100 g magnesium for the infancy period. It is often seen in the developing world that babies are nutrient deficient, although fed sufficiently (1). Conversely, lactation acts as a source of magnesium excretion and can precipitate maternal magnesium depletion if an excess volume of milk is produced (28).
Rare conditions
Understanding the functioning of regulatory magnesium proteins can help clarify how congenital conditions can develop due to an imbalance in both isolated magnesium conditions and with salt wasting tubulopathies (Table 2). Mutations in the TRPM6 genes have been identified in an autosomal recessive disorder called hypomagnesaemia with secondary hypocalcaemia which can present from infancy to adulthood depending on the severity of mutation (6,29). Autosomal dominant KCNA1 gene mutations result in Kv1.1 K+ channel defects. Isolated dominant hypomagnesaemia with hypocalciuria (IDH) is caused by mutations in the FXYD2 gene, leading to a mutant γ-subunit of the Na+-K+-ATPase protein (30). Patients present from young ages with these syndromes with severe hypomagnesaemia, tetany, muscle weakness, cramps and tremor (2).
Table 2
| Condition | Mutations |
|---|---|
| Renal magnesium wasting condition | |
| Familial primary hypomagnesaemia (and familial primary hypomagnesaemia with secondary hypocalcaemia) | CLDN16, CLDN19, FXYD2, HNF1B, CNNM2, EGF, KCNA1 (Kv1.1), TRPM6 |
| Bartter syndrome (type I, II, III and V) | SLC12A1 (NKCC2), KCNJ1 (ROMK), BSND (ClC-Kb), CASR |
| Gitelman syndrome | SLC12A3 (NCC) |
| EAST (epilepsy, ataxia, sensorineural deafness, and tubulopathy)/SeSAME (seizures, sensineural deafness, ataxia, mental retardation, and electrolyte imbalance) | KCNJ10 (Kir4.1) |
| Renal cysts and diabetes syndrome | HNF1-B |
| Autosomal dominant hypocalcaemia | CASR, GNA11 |
| Increased renal magnesium absorption | |
| Familial hypocalciuric hypercalcaemia | CASR, GNA11, AP2S1 |
The seizures, sensorineural deafness, ataxia, mental retardation and electrolyte imbalance syndrome, or SESAME (also known as EAST, for epilepsy, ataxia, sensorineural deafness and tubulopathy) syndrome, is a hypomagnesaemia disorder caused by mutations in KCNJ10 gene expressing the Kir4.1 K+ channel. Patients experience electrolyte abnormalities and hypomagnesaemic symptoms, as well as deafness, neurological manifestations, seizures and ataxia (2).
Familial hypomagnesaemia with hypercalciuria and nephrocalcinosis result from mutations in the claudin-16 and -19 genes (31,32). Renal failure, nephrocalcinosis, polyuria and polydipsia are some of the features described.
Thiazide diuretics inhibit the Na+-Cl− cotransporter (NCC/SLC12A3) which prevents sodium and magnesium reabsorption in the DCT, a process mimicked by Gitelman syndrome caused by a mutant NCC protein (2). Similarly, Bartter syndrome occurs with mutations in the NKCC2 and ROMK/KCNJ1 transport proteins, preventing magnesium reabsorption. These tubulopathies cause hypokalaemic metabolic alkalosis and can present from young ages including with failure to thrive and short stature.
CASR regulates PTH secretion based on the blood ionised calcium concentration. Autosomal dominant hypoparathyroidism (ADH), or familial hypocalciuric hypercalcemia with hypomagnesaemia, occurs when there are activating mutations in the CASR. Conversely, inactivating CASR mutations cause familial hypocalciuric hypercalcaemia, and patients experience hypermagnesaemia. In neonatal severe hyperparathyroidism (NSHPT) with inactivating CASR mutations, patients often require a total parathyroidectomy to control for the severe hypercalcaemia with hypermagnesaemia (23).
Epidermal growth factor (EGF) is needed for the absorption of magnesium in the DCT. Colon cancer patients treated with an anti-EGF antibody cetuximab have been shown to develop hypomagnesaemia from renal magnesium wasting (2).
Conclusions
Confirming the presence of hypomagnesaemia in particular can be challenging with multiple factors affecting magnesium concentration but not necessarily total body status. Diagnostic algorithms have been presented that are hoped to support users by providing an approach to diagnosing the cause of hypomagnesaemia and hypermagnesaemia. It is worth noting that magnesium may not be affected in isolation and use of related algorithms may further support the diagnosis (e.g., see acid base and potassium algorithms within this series). These algorithms cannot replace specialist knowledge, experience, and local guidelines but aim to support systematic investigation.
Acknowledgments
We would like to thank Professor Rousseau Gama for inspiring and inviting us to prepare this paper. All figures were created with BioRender.com.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Laboratory and Precision Medicine for the series “Investigative Algorithms in Laboratory Medicine – Electrolytes and Acid/Base”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-22-6/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-22-6/coif). The series “Investigative Algorithms in Laboratory Medicine – Electrolytes and Acid/Base” was commissioned by the editorial office without any funding or sponsorship. KS served as the unpaid Guest Editor of the series. ARS serves as Editor-in-Chief of Journal of Clinical and Experimental Dermatology. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fiorentini D, Cappadone C, Farruggia G, et al. Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Nutrients 2021;13:1136. [Crossref] [PubMed]
- de Baaij JH, Hoenderop JG, Bindels RJ. Regulation of magnesium balance: lessons learned from human genetic disease. Clin Kidney J 2012;5:i15-24. [Crossref] [PubMed]
- Huijgen HJ, Soesan M, Sanders R, et al. Magnesium levels in critically ill patients; what should we measure? Am J Clin Pathol 2000;114:688-95. [Crossref] [PubMed]
- Franken GAC, Adella A, Bindels RJM, et al. Mechanisms coupling sodium and magnesium reabsorption in the distal convoluted tubule of the kidney. Acta Physiol (Oxf) 2021;231:e13528. [Crossref] [PubMed]
- Yamanaka R, Shindo Y, Oka K. Magnesium Is a Key Player in Neuronal Maturation and Neuropathology. Int J Mol Sci 2019;20:3439. [Crossref] [PubMed]
- Houillier P. Mechanisms and regulation of renal magnesium transport. Annu Rev Physiol 2014;76:411-30. [Crossref] [PubMed]
- Kroll MH, Elin RJ. Relationships between magnesium and protein concentrations in serum. Clin Chem 1985;31:244-6. [Crossref] [PubMed]
- Scarpati G, Baldassarre D, Oliva F, et al. Ionized or Total Magnesium levels, what should we measure in critical ill patients? Transl Med UniSa 2020;23:68-76. [PubMed]
- Nielsen FH, Johnson LA. Data from Controlled Metabolic Ward Studies Provide Guidance for the Determination of Status Indicators and Dietary Requirements for Magnesium. Biol Trace Elem Res 2017;177:43-52. [Crossref] [PubMed]
- Gullestad L, Dolva LO, Waage A, et al. Magnesium deficiency diagnosed by an intravenous loading test. Scand J Clin Lab Invest 1992;52:245-53. [Crossref] [PubMed]
- Agus ZS. Hypomagnesemia. J Am Soc Nephrol 1999;10:1616-22. [Crossref] [PubMed]
- Ayuk J, Gittoes NJ. How should hypomagnesaemia be investigated and treated? Clin Endocrinol (Oxf) 2011;75:743-6. [Crossref] [PubMed]
- Bowen RA, Remaley AT. Interferences from blood collection tube components on clinical chemistry assays. Biochem Med (Zagreb) 2014;24:31-44. [Crossref] [PubMed]
- Hardwick LL, Jones MR, Brautbar N, et al. Magnesium absorption: mechanisms and the influence of vitamin D, calcium and phosphate. J Nutr 1991;121:13-23. [Crossref] [PubMed]
- Blaine J, Chonchol M, Levi M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin J Am Soc Nephrol 2015;10:1257-72. [Crossref] [PubMed]
- Ryan MP. Interrelationships of magnesium and potassium homeostasis. Miner Electrolyte Metab 1993;19:290-5. [PubMed]
- Whang R, Ryder KW. Frequency of hypomagnesemia and hypermagnesemia. Requested vs routine. JAMA 1990;263:3063-4. [Crossref] [PubMed]
- Clark BA, Brown RS. Unsuspected morbid hypermagnesemia in elderly patients. Am J Nephrol 1992;12:336-43. [Crossref] [PubMed]
- Schelling JR. Fatal hypermagnesemia. Clin Nephrol 2000;53:61-5. [PubMed]
- Walker P, Parnell S, Dillon RC. Epsom Salt Ingestion Leading to Severe Hypermagnesemia Necessitating Dialysis. J Emerg Med 2020;58:767-70. [Crossref] [PubMed]
- Glendenning P. Summary statement from a workshop on asymptomatic primary hyperparathyroidism: a perspective for the 21st century. Clin Biochem Rev 2003;24:27-30.
- Larsson A, Palm M, Hansson LO, et al. Reference values for clinical chemistry tests during normal pregnancy. BJOG 2008;115:874-81. [Crossref] [PubMed]
- Abbassi-Ghanavati M, Greer LG, Cunningham FG. Pregnancy and laboratory studies: a reference table for clinicians. Obstet Gynecol 2009;114:1326-31. [Crossref] [PubMed]
- Teasdale S, Morton A. Changes in biochemical tests in pregnancy and their clinical significance. Obstet Med 2018;11:160-70. [Crossref] [PubMed]
- Zarean E, Tarjan A. Effect of Magnesium Supplement on Pregnancy Outcomes: A Randomized Control Trial. Adv Biomed Res 2017;6:109. [Crossref] [PubMed]
- Kolanu BR, Vadakedath S, Boddula V, et al. Activities of Serum Magnesium and Thyroid Hormones in Pre-, Peri-, and Post-menopausal Women. Cureus 2020;12:e6554. [Crossref] [PubMed]
- Adeli K, Higgins V, Trajcevski K, et al. The Canadian laboratory initiative on pediatric reference intervals: A CALIPER white paper. Crit Rev Clin Lab Sci 2017;54:358-413. [Crossref] [PubMed]
- Greenwald JH, Dubin A, Cardon L. Hypomagnesemic tetany due to excessive lactation. Am J Med 1963;35:854-60. [Crossref] [PubMed]
- Orphan net. Familial primary hypomagnesemia | Genetic and Rare Diseases Information Center (GARD) – an NCATS Program (nih.gov). Available online: https://rarediseases.info.nih.gov/diseases/2906/familial-primary-hypomagnesemia. Accessed Jan 22, 2022.
- Konrad M, Schlingmann KP, Gudermann T. Insights into the molecular nature of magnesium homeostasis. Am J Physiol Renal Physiol 2004;286:F599-605. [Crossref] [PubMed]
- OMIM Entry. # 248250 - Hypomagnesemia 3, renal; HOMG3. Available online: https://www.omim.org/entry/248250. Accessed Apr 27, 2022.
- OMIM Entry. # 248190 - Hypomagnesemia 5, renal, with or without ocular involvement; HOMG5. Available online: https://omim.org/entry/248190. Accessed Apr 27, 2022.
Cite this article as: Darragh-Hickey C, Kaur S, Flowers KC, Allen GT, Shipman AR, Shipman KE. Investigative algorithms for disorders affecting plasma magnesium: a narrative review. J Lab Precis Med 2022;7:21.