
COVID-19 vaccination in patients taking immunosuppressant drugs: what is the best strategy for improving immunogenicity?
The apparently unstoppable coronavirus disease 2019 (COVID-19) pandemic is still causing paramount clinical, economical and social derangements (1). Besides the widespread adoption of physical preventive measures that seems partially effective for reducing the likelihood of being infected by this novel coronavirus, it is now undeniable that universal vaccination against COVID-19 must be seen as a mainstay for preventing virus propagation and for limiting the risk of developing severe and/or critical COVID-19 illness (2), especially in the more vulnerable parts of the population (3). While the efficacy of COVID-19 vaccination remains considerably high, reportedly better than that of influenza vaccination, even against new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern (VOCs) like Omicron BA.2.75, BA.4 and BA.5 (4), vaccine response is inadequate in certain categories of patients (5).
Among the various conditions that may strongly influence the immunogenicity of COVID-19 vaccines, immunosuppression (both pathological and drug-induced) plays a key role. Several lines of evidence now attest that patients taking a kaleidoscope of immunosuppressant agents like steroids, mycophenolate, methotrexate, anti-tumor necrosis factor (TNF) drugs, anti-CD20 treatments (e.g., rituximab) and infliximab (among others) display considerably lower vaccine immunogenicity, as reflected by a blunted generation of anti-SARS-CoV-2 neutralizing antibodies compared to people not receiving these medications (6). This is indeed a relevant clinical issue, since these already fragile patients may be exposed to a dramatically enhanced risk of developing severe symptoms due to the strong relationship between lower levels of anti-SARS-CoV-2 neutralizing antibodies and unfavourable disease progression (7). A crucial aspect thus emerges concerning the identification of the most effective and safe means to boost anti-SARS-CoV-2 neutralizing antibodies response in these people.
Based on the current evidence, at least three potential approaches could be advocated, as summarized in Figure 1, all characterized by their own specific limitations. The first strategy entails a temporary suspension (usually between 1–2 weeks) of immunosuppressant treatment upon receiving COVID-19 vaccination. Araujo et al. conducted a prospective, randomised intervention study (8), where 129 patients with rheumatoid arthritis on methotrexate therapy were randomized to either withhold the drug for 2 weeks after primary COVID-19 vaccination (CoronaVac) or maintain their actual dosage. Nearly 2 months after vaccination, patients who suspended the immunosuppressant therapy had an anti-SARS-CoV-2 antibodies titer nearly twice as high as those who did not pause the treatment, while reporting a similar flare rate and disease activity score. In a subsequent retrospective study, Arumahandi de Silva et al. enrolled 64 patients with autoimmune rheumatic diseases on methotrexate therapy (9), who were randomized to pause the treatments for at least 7 days in concomitance with COVID-19 vaccination (BNT162b2, mRNA-1273 or AZD1222) or continue the therapy. As predicted, patients who discontinued methotrexate had significantly higher values (nearly double) of anti-SARS-CoV-2 neutralising antibodies compared to those who did not, especially those aged 60 years or older. No safety endpoints were assessed in this trial. More recently, Abhishek et al. published the results of a multicentre, prospective, randomised trial including 254 patients with immune-mediated inflammatory disease under methotrexate treatment (10). Patients randomized to suspend the drug for 2 weeks after receiving a COVID-19 vaccine booster (BNT162b2, mRNA-1273 or AZD1222) displayed anti-SARS-CoV-2 antibodies levels that were nearly double compared to those who did not temporarily withhold methotrexate. No serious adverse events were reported in either group.
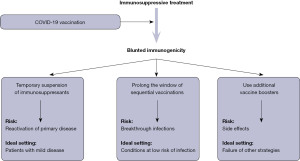
Unlike a strategy based on drug suspension, a possible alternative to boost vaccine immunogenicity entails the modification of standard vaccination protocols, either in terms of time between boosters or alterations in dosage. Mehta et al. studied 495 patients with autoimmune rheumatic diseases taking immunosuppressant drugs (methotrexate, sulfasalazine or mycophenolate) (11), who were randomized to receive two AZD1222 vaccine doses at either 4–6- or 10–14-week interval. Notably, patients with delayed administration of the second vaccine dose displayed 80% higher anti-SARS-CoV-2 antibodies levels compared to those who underwent the conventional vaccination cycle, while exhibiting a nearly identical rate of breakthrough infections. In another investigation, Midtvedt et al. administrated a fourth mRNA vaccine dose in 188 kidney transplant immunosuppressed recipients previously classified as low-responders (12). Interestingly, nearly half (i.e., 42%) of such patients developed a sustained anti-SARS-CoV-2 neutralizing antibodies response after the fourth vaccine dose. Similar results were obtained in a separate trial published by Teles et al. and based on 18 patients taking immunosuppressant drugs who previous had a modest immunogenic response to two mRNA COVID-19 vaccine doses (13). In this study, the administration of the fourth COVID-19 vaccine dose was effective to increase the anti-SARS-CoV-2 antibodies response, reaching the positivity threshold in 67% of patients, with no clinically significant adverse events. An even more interesting study has recently been published by Osmanodja et al. Briefly, the authors studied nearly 900 kidney transplant recipients taking a variety of immunosuppressive drugs, who received three, four or five doses of COVID-19 vaccines (BNT162b2, mRNA-1273, AZD1222 or Ad26.COV2.S) (14). The serological response rate increased steadily across the different vaccination cohorts, from <50% in those with three COVID-19 vaccine doses, to 75% in those with four doses, up to nearly 90% in those with five doses. Notably, in a limited cohort of patients who discontinued the immunosuppressive treatment, the rate of anti-SARS-CoV-2 seropositivity was 75% compared to nearly 50% in those who reduced the dosage or continued the therapy, thus suggesting that partial immunosuppressant dose reduction does not seem a suitable approach.
The results of these important studies would hence suggest that all the three potential strategies (as summarized in Figure 1) may be effective and safe to boost the immunogenic response in patients taking immunosuppressant drugs. Indeed, the risks of each of these approaches cannot be discounted, since the trials completed and published so far were actually based on a limited sample of patients and would hence require further validation in larger cohorts. In particular, temporary suspension of immunosuppressant therapy may predispose to reactivation of primary autoimmune disease. while prolonging the window of vaccine dose administration may ad interim increase the risk of breakthrough infections. Finally, repeated administration of vaccine boosters may be associated with higher risk of developing side effects.
Therefore, the use of a personalized approach, individualizing the relative risk of each strategy according to environmental risk and clinical aspects (Figure 1), may be advisable at this point in time, at least until more solid evidence will provide support for the superiority of one strategy over the others.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was a standard submission to the journal. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-22-49/coif). GL serves as the Editor-in-Chief of Journal of Laboratory and Precision Medicine. The other authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Arsenault C, Gage A, Kim MK, et al. COVID-19 and resilience of healthcare systems in ten countries. Nat Med 2022;28:1314-24. [Crossref] [PubMed]
- Mattiuzzi C, Lippi G. COVID-19 vaccines efficacy in preventing or limiting SARS-CoV-2 infections. J Infect 2022;84:722-46. [Crossref] [PubMed]
- Mattiuzzi C, Henry BM, Lippi G. COVID-19 vaccination uptake strongly predicts averted deaths of older people across Europe. Biomed J 2022; Epub ahead of print. [Crossref] [PubMed]
- Monto AS. The Future of SARS-CoV-2 Vaccination - Lessons from Influenza. N Engl J Med 2021;385:1825-7. [Crossref] [PubMed]
- Lippi G, Henry BM, Plebani M. Optimizing effectiveness of COVID-19 vaccination: will laboratory stewardship play a role? Clin Chem Lab Med 2021;59:1885-8. [Crossref] [PubMed]
- Jena A, Mishra S, Deepak P, et al. Response to SARS-CoV-2 vaccination in immune mediated inflammatory diseases: Systematic review and meta-analysis. Autoimmun Rev 2022;21:102927. [Crossref] [PubMed]
- Feng S, Phillips DJ, White T, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med 2021;27:2032-40. [Crossref] [PubMed]
- Araujo CSR, Medeiros-Ribeiro AC, Saad CGS, et al. Two-week methotrexate discontinuation in patients with rheumatoid arthritis vaccinated with inactivated SARS-CoV-2 vaccine: a randomised clinical trial. Ann Rheum Dis 2022;81:889-97. [Crossref] [PubMed]
- Arumahandi de Silva AN, Frommert LM, Albach FN, et al. Pausing methotrexate improves immunogenicity of COVID-19 vaccination in elderly patients with rheumatic diseases. Ann Rheum Dis 2022;81:881-8. [Crossref] [PubMed]
- Abhishek A, Boyton RJ, Peckham N, et al. Effect of a 2-week interruption in methotrexate treatment versus continued treatment on COVID-19 booster vaccine immunity in adults with inflammatory conditions (VROOM study): a randomised, open label, superiority trial. Lancet Respir Med 2022; Epub ahead of print. [Crossref] [PubMed]
- Mehta P, Paul A, Ahmed S, et al. Effectiveness of delayed second dose of AZD1222 vaccine in patients with autoimmune rheumatic disease. Clin Rheumatol 2022; Epub ahead of print. [Crossref] [PubMed]
- Midtvedt K, Vaage JT, Heldal K, et al. Fourth dose of the SARS-CoV-2 vaccine in kidney transplant recipients with previously impaired humoral antibody response. Am J Transplant 2022; Epub ahead of print. [Crossref] [PubMed]
- Teles M, Connolly CM, Frey S, et al. Attenuated response to fourth dose SARS-CoV-2 vaccination in patients with autoimmune disease: a case series. Ann Rheum Dis 2022;81:738-40. [Crossref] [PubMed]
- Osmanodja B, Ronicke S, Budde K, et al. Serological Response to Three, Four and Five Doses of SARS-CoV-2 Vaccine in Kidney Transplant Recipients. J Clin Med 2022;11:2565. [Crossref] [PubMed]
Cite this article as: Lippi G, Mattiuzzi C, Henry BM. COVID-19 vaccination in patients taking immunosuppressant drugs: what is the best strategy for improving immunogenicity? J Lab Precis Med 2022;7:29.


