
Cut-off points of 75-gram oral glucose tolerance test as a diagnostic test for gestational diabetes mellitus in pregnant Filipino population
Introduction
Laboratory testing is a huge contributory component in clinical decision making. It is used in the diagnosis of diseases, therapeutic monitoring, staging of disease, and even disease risk prediction. A critical phase in laboratory testing is the use medical decision points. Medical decision point was first put into theory in 1960 by Schneider in his paper entitled, “Some thoughts on normal, or standard, values in clinical medicine” (1). Although the theory has been set forth 50 years ago, its application is still not fully complete today because of the following challenges: (I) lack of standardization of analytical methods; (II) population and racial based variations in laboratory values; (III) difficulty in establishing cut-off points; (IV) lack of reference population for establishing cut-off points; (V) high time and cost requirement, and among others. Because of these limitations, the practical usefulness of cut-off points is more often lower than its actual theoretical power (2).
Establishing cut-off points or medical decision limits is a demanding activity, but it is necessary to ensure that clinical decision making is reliable. A good set of cut-off points must consider the population serviced by the laboratory, that is, the reference population used to establish the limits should have similar characteristics with that of the patients catered by the laboratory. A previous study has mentioned cut-off points are not universal, and that it should be identified per region and per condition (3). As cut-off points are greatly affected by various factors, it is essential to establish a cut-off point that is suitable for a specific population of individuals. Well established cut-off points for almost all the analytes measured in the laboratory are available. However, verification in terms of its applicability on the population serviced by the laboratory must be performed. Without verification, its use will lead to inaccurate interpretation of results, and, consequently, erroneous clinical decisions.
One good and common example of inaccurate interpretation of results due to unverified cut-off points is in the interpretation of the diagnostic tool utilized for gestational diabetes mellitus (GDM) among the Filipino pregnant women. GDM is a form of hyperglycemia recognized on the onset of pregnancy and its prevalence is increasing worldwide (4). Although it is a transient condition, it causes short- and long-term complications both to the mother and the baby. GDM is now a major health problem affecting maternal and fetal health worldwide. The GDM pregnancy condition is diagnosed using 75-grams (g) oral glucose tolerance test (OGTT) (5). Aside from 75-g OGTT, there are other laboratory tests available and recommended to assess hyperglycemia in pregnancy such as fasting blood glucose (FBG), glycosylated hemoglobin (HbA1c), oral glucose challenge test, and OGTT using different glucose loads (6,7), but the use of OGTT is the gold standard in GDM diagnosis.
The results of OGTT are interpreted by following a certain criteria or cut-off points. Unfortunately, there are numerous recommended criteria or cut-off points for OGTT interpretation, namely: the World Health Organization (WHO) criteria, American Diabetes Association (ADA) criteria, International Association of Diabetes and Pregnancy Study Group (IADPSG) criteria, American College of Obstetrics and Gynecologists (ACOG), Carpenter and Coustan, National Diabetes Data Group (NDDG), Philippine Obstetrical and Gynecological Society (POGS) to name a few (8-10). Table 1 summarizes the most common diagnostic criteria utilized in interpreting 75-g OGTT in the Philippines. With the numerous available diagnostic criteria, physicians are confused on which criteria to follow in diagnosing GDM, thus causing misdiagnosis (11). On one hand, the use of a stricter diagnostic criteria such as that of ACOG and ADA criteria, requiring to exceed two threshold values to be diagnosed as GDM, may cause a lower prevalence of GDM but may result to un-diagnosis of the disease that should be treated. On the other hand, the use of POGS criteria, which is more lenient as it requires to exceed one out of two threshold values only, may cause higher GDM prevalence but may over-diagnose pregnant women, thereby giving psychological and financial burden to the mothers (11-13).
Table 1
| Glucose in mmol/L | WHO* | IADPSG* | ADA** | POGS* |
|---|---|---|---|---|
| FBS | 7.0 | 5.1 | 5.28 | >5.1 |
| 1st hour glucose | − | 10.0 | 10.0 | |
| 2nd hour glucose | 11.1 | 8.5 | 8.61 | ≥7.8 |
These were the old WHO and ADA criteria. Recently, WHO and ADA already recommended the use of IADPSG criteria (3,4). *, one threshold should be met to diagnose GDM; **, two thresholds should be met to diagnose GDM. g, grams; OGTT, oral glucose tolerance test; WHO, World Health Organization; IADPSG, The International Association of Diabetes and Pregnancy Study Groups; ADA, American Diabetes Association; POGS, Philippine Obstetrical and Gynecological Society; FBS, fasting blood sugar; GDM, gestational diabetes mellitus.
In the Philippines, since we are following various cut-off points provided by foreign organizations, these cut-off points were not verified by its reference population. The criteria utilized to interpret 75-g OGTT vary and depend on physicians’ preference. Increasing or decreasing the threshold of the diagnostic criteria has an impact to pregnant women, physically and financially. Thus, cut-off points for 75-g OGTT must be verified and established based on Filipino reference population to improve GDM diagnosis. We present the following article in accordance with the STARD reporting checklist (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-22-26/rc).
Methods
Study design
This is a population-based prospective study of GDM in Metro Manila, Philippines done to establish medical decision limits or cut-off points of OGTT for the diagnosis of GDM. A total of 918 pregnant women were gathered and provided with comprehensive questionnaires upon consent to participate in the study. Demographic and biochemical profiling were performed to identify and select accepted reference sample group. Standard information such as age, height, weight, and blood pressure (BP) were obtained from the participant. Inclusion of participants was limited to healthy pregnant adults aged 18 to 45 years old as identified by their attending physicians. Individuals with any form of diabetes, with inflammatory conditions, and metabolic diseases such as polycystic ovarian syndrome and obesity were excluded in the reference sample group. From these selection criteria, out of 918 potential subjects, we only included 469 pregnant women in the reference sample group in order to establish cut-off points for 75-g OGTT for the diagnosis of GDM. The number of accepted and excluded participants as reference sample group are illustrated in the study flowchart shown in Figure 1. To remove potential variation due to age of gestation (AOG) when OGTT was performed, reference sample group was categorized into two: early OGTT group, those who had their OGTT taken before 28 weeks of gestation, and late OGTT group, those who had their OGTT taken beyond 28 weeks of gestation. No further categorization of subjects was done based on gender and age because we only recruited female, pregnant, and child-bearing age population.
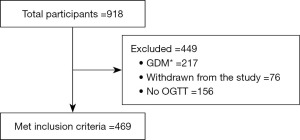
Prior to recruitment, protocol of this study was reviewed and approved by the University of Santo Tomas Graduate School Ethics Review Committee with Protocol Number E-2016-02-R3. Study was implemented from 2018 to 2020, with ethics approval being renewed yearly. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was taken from all the patients.
Data and resource availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request. Similarly, the resource generated during and/or analyzed during the current study is also available from the corresponding author upon reasonable request.
Blood collection, handling and testing
Blood samples were collected by registered medical technologists trained to perform phlebotomy. For analytes due for serum testing, samples were placed into serum separator tubes. For analytes due for whole blood testing, samples were placed in tubes with dipotassium ethylene diamine tetra acetic acid (K2EDTA). Blood in serum separator tube was allowed to clot for 15 minutes prior to spinning at 2,500 RPM for 15 minutes in a non-refrigerated centrifuge machine (Beckman Coulter, Allegra X-30, Brea, California, USA). Separated serum samples were immediately processed to measure required analytes.
Testing: glucose, lipid profile, glycosylated hemoglobin, and insulin
Measurement of glucose, total cholesterol, triglyceride, and high density lipoprotein utilized colorimetric enzymatic assay following manufacturer’s protocol (Human Liquicolor® reagents, Wiesbaden, Germany). Glycosylated hemoglobin was measured using NycocardTM HbA1c. While for insulin, enzyme linked immunosorbent assay was performed (RayBio® Human Insulin kit, Georgia, USA). Homeostatic model assessment for insulin resistance (HOMA-IR) was computed following the formula: fasting blood sugar (FBS) (mmol/L) × insulin (uIU/mL)/22.5.
OGTT
The standard 75-gram OGTT procedure was followed in this study. Briefly, consented participants were instructed to fast overnight prior to the day of blood collection. Upon arrival at the recruitment area at the hospital, fasting blood specimen was collected; then, 75-g glucose load was given. First hour and second hour blood specimens were collected one hour and two hours after the start of drinking the glucose load (14).
Definition of criteria
Diagnosis of GDM in the Philippines varies in terms of laboratory tests utilized and on the cut-off points used to interpret the laboratory results. Four criteria are being utilized in the Philippines to interpret 75-g OGTT, namely; WHO, IADPSG, ADA, and POGS criteria (13).
In this study, we utilized the IADPSG criteria to analyze and interpret OGTT results of the recruited participants and select the reference sample group (FBS, 5.1 mmol/L; 1st hour glucose, 10.0 mmol/L; 2nd hour glucose, 8.5 mmol/L). IADPSG criteria is being recommended by WHO for the diagnosis of GDM. Only those with normal OGTT results were included in the reference sample group. Body mass index (BMI) was calculated based on self-reported height and weight, and it was interpreted following The Royal Women’s Hospital (Victoria, Australia) recommendation for the Asian population. BMI of reference sample group was interpreted following these criteria: underweight, BMI <18.5 kg/m2; normal weight, 18.5–22.9 kg/m2; overweight, 23.0–27.5 kg/m2; and obese, ≥27.5 kg/m2.
Statistical analyses
Using the 29% prevalence of GDM in the Philippines published in 2018 (11), with precision of 0.05, the computed minimum sample size requirement for this study is 316. Although most references mentioned the minimum requirement of 120 individuals for the establishment of medical decision limits (15), we were able to include a total of 469 healthy individuals. In previous studies, a minimum range between 300 to 400 individuals is said to be sufficiently large in ensuring a statistically significant and reliable cut-off points (16,17). Descriptive statistics was used to analyze demographic and biochemical variables and are presented as mean ± standard error of mean (SEM). After carefully defining and selecting the reference sample group and measuring 75-g OGTT and other biochemical tests, the statistical analyses of the results of the 469 pregnant women that passed all criteria for establishing cut-off points were performed. Mean, and median values of the variables were presented. Unpaired t-test was utilized to compare OGTT results of the reference sample group categorized based on the AOG of glucose testing. The collected data were used to construct the histogram to show the normal distribution of the data, and parametric method was applied. Area under the receiver operating characteristics (ROC) was prepared in comparison with the IADPSG criteria. From the area under the ROC curve, we derived three cut-off points (a, b, and c) following suggested methodologies in selecting appropriate cut-off points from previous studies (3,18-20). The identified three cut-off points were applied and diagnostic efficiency of the three were compared to identify the most appropriate cut-off point and the number of threshold values to be used in diagnosing GDM among the Filipino population. Prism 9.0 software for macOS (GraphPad Software, San Diego, CA, USA) was utilized in the data analyses.
Results
Characteristics of reference sample group and sources of variation
This study included a total of 469 healthy pregnant women as reference sample group. Table 2 presents demographic and biochemical variables such as age, weight, height, BMI, BP, lipid profile, glycosylated hemoglobin, insulin, and HOMA-IR of the healthy pregnant women. All biochemical parameters are within the acceptable cut-off points per analyte.
Table 2
| Variables | Median (IQR) | Mean ± SEM |
|---|---|---|
| Age, years | 26 (22.0–31.0) | 27±0.278 |
| Pre-pregnancy weight, k | 52 (45.0–60.0) | 53.7±0.544 |
| Height, m | 1.55 (1.52–1.59) | 1.56±0.003 |
| Pre-pregnancy BMI, kg/m2 | 21.5 (19.2–24.4) | 22.1±0.224 |
| Pre-BMI Classification | 4 (4.0–5.0) | 4±0.048 |
| Gestational weight, k | 56 (50.6–62.0) | 57.0±0.492 |
| Gestational BMI, kg/m2 | 23.3 (20.9–25.9) | 23.4±0.215 |
| Gestational BMI Classification | 5 (4.0–5.0) | 5±0.048 |
| Age of gestation, weeks | 27 (23.0–32.0) | 27.4±0.282 |
| SBP, mmHg | 100 (90.0–110.0) | 100±0.661 |
| DBP, mmHg | 70 (60.0–75.0) | 70±0.521 |
| Total cholesterol, mmol/L | 4.76 (4.04–5.81) | 5.05±0.072 |
| Triglyceride, mmol/L | 2.35 (1.78–3.22) | 2.60±0.058 |
| HDL-C, mmol/L | 1.74 (1.50–2.07) | 1.81±0.022 |
| LDL-C, mmol/L | 1.92 (1.23–2.86) | 2.13±0.066 |
| HbA1c, % (mmol/mol) | 5.1 (4.6–5.4); 32 (27.0–36.0) | 5.1±0.031 (32±0.403) |
| Insulin, uIU/mL | 6.79 (3.66–10.8) | 8.12±0.351 |
| HOMA-IR | 1.31 (0.72–2.07) | 1.53±0.066 |
Interpretation of BMI: normal BMI, 18.5–22.9. BMI Classification: 3, underweight; 4, normal weight; 5, overweight; and 6, obese. IQR, interquartile range; SEM, standard error of mean; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL-C, high density lipoprotein-cholesterol; LDL-C, low density lipoprotein-cholesterol; HbA1c, glycosylated hemoglobin; HOMA-IR, homeostatic model assessment for insulin resistance.
Other potential sources of OGTT result variability
Since studies have shown variability of glucose measurement depending on AOG, it is important to remove this source of variability in the establishment of cut-off points (21,22). In this present study, blood specimens were collected in pregnant women based on the recommendations of their attending physicians, which made AOG a source of variability. Table 3 presents OGTT results of the reference sample group categorized based on the AOG when OGTT was done. This comparison was made to remove AOG as potential source of variability in establishing the cut-off points.
Table 3
| Variable | Glucose levels, mmol/L | P value# | |
|---|---|---|---|
| Early OGTT (mean ± SEM) | Late OGTT (mean ± SEM) | ||
| FBS | 4.30±0.03 | 4.29±0.06 | 0.9792 |
| 1st hour glucose | 6.63±0.08 | 6.82±0.15 | 0.3124 |
| 2nd hour glucose | 5.88±0.06 | 5.93±0.12 | 0.6350 |
Early OGTT means OGTT done on or before 28 weeks of gestation; late OGTT means OGTT done beyond 28 weeks of gestation. #, P value >0.05 indicates no significant difference in OGTT results between early and late OGTT. OGTT, oral glucose tolerance test; SEM, standard error of mean; FBS, fasting blood sugar.
OGTT Results and histogram analysis
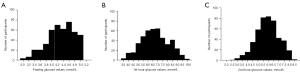
Histogram was prepared following a parametric method of analysis after removing the outlier data using robust regression and outlier removal (ROUT) method with Q or maximum desired false discovery rate (FDR) of 1%. Table 4 shows the OGTT results of the reference sample group with skewness, kurtosis, and the lower and upper 95% CI of mean. Based on skewness values, we can say that the data we collected are fairly symmetrical, thus we have a normal distribution of data. Similarly, kurtosis is near zero, indicating that the data are normally distributed. To better visualize the distribution of data, histograms of the OGTT results are shown in Figure 2.
Table 4
| Variable | Mean glucose values, mmol/L ± SEM | Skewness | Kurtosis | Lower 95% CI of mean | Upper 95% CI of mean |
|---|---|---|---|---|---|
| FBS | 4.30±0.022 | −0.532 | −0.163 | 4.25 | 4.34 |
| 1-hour glucose | 6.70±0.064 | 0.045 | −0.549 | 6.57 | 6.82 |
| 2-hour glucose | 5.91±0.050 | −0.178 | −0.174 | 5.81 | 6.01 |
If the skewness is between −0.5 and 0.5, the data are fairly symmetrical; if the skewness is between −1 and −0.5 or between 0.5 and 1, the data are moderately skewed; If the skewness is less than −1 or greater than 1, the data are highly skewed; kurtosis presented here is excess kurtosis. SEM, standard error of mean; CI, confidence interval.
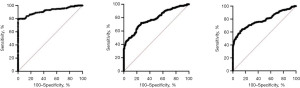
Area under the ROC curve and measures of diagnostic efficiency of the proposed cut-off points
After identifying the reference sample group, cut-off points for OGTT were derived from area under curve following several criteria in determining the most appropriate cut-off point (Table 5 and Figure 3). Various studies have identified ways to determine the most appropriate cut-off values (18,19,23). Some of which include the use of the point in the ROC where sensitivity equals specificity, the use of Youden’s index, the use of the point where sensitivity is at least 80%, and the use of the point on the curve with minimum distance from the upper left corner of the unit square, among others (3). In this present study, we utilized three methods to identify cut-off points: (I) use of the point in the ROC curve where sensitivity = specificity; (II) use of the point in the ROC curve where sensitivity is at least 80%; and (III) use of the point in the ROC curve with minimum distance from the upper left corner of the square. We labelled these three methods in the text as superscripts a, b, and c.
Table 5
| Parameters | AUC (95% CI) | Cut-off pointa | Cut-off pointb | Cut-off pointc | P value* |
|---|---|---|---|---|---|
| FBS | 0.9183 (0.8912–0.9454) | >4.8 | >4.9 | >4.9 | <0.0001 |
| 1st hour blood glucose | 0.7813 (0.7421–0.8204) | >7.5 | >6.6 | >7.2 | <0.0001 |
| 2nd hour blood glucose | 0.7844 (0.7433–0.8256) | >6.6 | >5.8 | >6.4 | <0.0001 |
*, P value <0.05 indicates that testing done can discriminate well GDM from non-GDM patients. a, use of the point in the ROC curve where sensitivity = specificity; b, use of the point in the ROC curve where sensitivity is at least 80%; c, use of the point in the ROC curve with minimum distance from the upper left corner of the square. FBS, fasting blood sugar; AUC, area under curve; CI, confidence interval; ROC, receiver operating characteristic; GDM, gestational diabetes mellitus.
Following the identification of proposed cut-offs points of fasting, first-hour, and second-hour blood glucose levels in 75-g OGTT, it is imperative to assess its corresponding diagnostic efficiency (Table 6). In this study, we utilized the IADPSG criteria as the standard diagnostic criteria in interpreting OGTT results to determine diagnostic efficiency parameters such as sensitivity, specificity, positive predictive and negative predictive values (NPVs). The use of IADPSG criteria gave a GDM prevalence of 31.6% in the Philippines (n=686). We also determined the number of threshold values that needs to be met to be considered as GDM.
Table 6
| Variables | Prevalence, % | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV, % (95% CI) | NPV, % (95% CI) |
|---|---|---|---|---|---|
| Cut-off pointa | |||||
| FBS alone | 38.34 | 100.0 (98.31–100.0) | 90.2 (87.13–92.73) | 82.5 (78.19–86.12) | 100.0 |
| 1st hour glucose alone | 41.25 | 100.0 (98.31–100.0) | 85.9 (82.45–88.95) | 76.7 (72.44–80.44) | 100.0 |
| 2nd hour glucose alone | 40.38 | 100.0 (98.31–100.0) | 87.2 (83.84–90.09) | 78.3 (74.06–82.08) | 100.0 |
| FBS + 1st hour | 3.94 | 12.4 (8.36–17.58) | 100.0 (99.22–100.0) | 100.0 | 71.2 (70.13–72.19) |
| FBS + 2nd hour | 3.79 | 12.0 (7.98–17.06) | 100.0 (99.22–100.0) | 100.0 | 71.1 (70.04–72.06) |
| 1st hour + 2nd hour | 11.22 | 35.5 (29.13–42.24) | 100.0 (99.22–100.0) | 100.0 | 77.0 (75.22–78.71) |
| FBS + 1st hour + 2nd hour | 18.51 | 58.5 (51.66–65.15) | 100.0 (99.22–100.0) | 100.0 | 83.9 (81.65–85.92) |
| Cut-off pointb | |||||
| FBS alone | 32.5 | 100.0 (98.31–100.0) | 98.7 (97.24–99.53) | 97.3 (94.23–98.77) | 100.0 |
| 1st hour glucose alone | 60.5 | 100.0 (98.31–100.0) | 57.8 (53.17–62.30) | 52.3 (49.64–54.92) | 100.0 |
| 2nd hour glucose alone | 62.5 | 100.0 (98.31–100.0) | 54.8 (50.17–59.37) | 50.6 (48.09–53.07) | 100.0 |
| FBS + 1st hour | 2.6 | 8.3 (4.99–12.79) | 100.0 (99.22–100.0) | 100.0 | 70.2 (69.37–71.04) |
| FBS + 2nd hour | 3.6 | 11.5 (7.6–16.54) | 100.0 (99.22–100.0) | 100.0 | 71.0 (69.95–71.93) |
| 1st hour + 2nd hour | 28.9 | 91.2 (86.66–94.65) | 100.0 (99.22–100.0) | 100.0 | 96.1 (94.14–97.43) |
| FBS + 1st hour + 2nd hour | 20.7 | 65.4 (58.70–71.75) | 100.0 (99.22–100.0) | 100.0 | 86.2 (83.89–88.25) |
| Cut-off pointc | |||||
| FBS alone | 32.5 | 100.0 (98.31–100.0) | 98.7 (97.24–99.53) | 97.3 (94.23–98.77) | 100.0 |
| 1st hour glucose alone | 46.6 | 100.0 (98.31–100.0) | 78.0 (74.01–81.70) | 67.8 (63.98–71.42) | 100.0 |
| 2nd hour glucose alone | 46.4 | 100.0 (98.31–100.0) | 78.5(74.46–82.10) | 68.2 (64.38–71.86) | 100.0 |
| FBS + 1st hour | 3.1 | 9.7 (6.09–14.41) | 100.0 (99.22–100.0) | 100.0 | 70.5 (69.61–71.42) |
| FBS + 2nd hour | 3.1 | 9.7 (6.09–14.41) | 100.0 (99.22–100.0) | 100.0 | 70.5 (69.61–71.42) |
| 1st hour + 2nd hour | 17.8 | 56.2 (49.34–62.93) | 100.0 (99.22–100.0) | 100.0 | 83.2 (80.94–85.16) |
| FBS + 1st hour + 2nd hour | 17.1 | 53.9 (47.04–60.69) | 100.0 (99.22–100.0) | 100.0 | 82.4 (82.56–87.98) |
Cut-off pointa: first method of cut-off point identification. It uses points in the ROC curve where sensitivity is equal to specificity; Cut-off pointb: second method of cut-off point identification. It uses points in the ROC curve where sensitivity is at least 80%; Cut-off pointc: third method of cut-off point identification. It uses points in the ROC curve with minimum distance from the upper left corner of the square. GDM, gestational diabetes mellitus; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value; FBS, fasting blood sugar.
Discussion
Laboratory testing for glucose plays a very important role in diagnosing GDM, as well as other types of diabetes. The 75-g OGTT is the standard test used in diagnosing GDM. However, it is an inconvenient test, time consuming, and faced with challenges in terms of result interpretation because of numerous criteria available to analyze the results (8,24). In this study, we wanted to identify and establish the cut-off points for 75-g OGTT that is most appropriate and applicable for Filipino pregnant women.
The mean age of the reference sample group is 26 years old, which is within the recommended age of pregnancy. Pregnancy age between 20 to 30 years old is recommended because women at these age range are less likely to be at risk of developing pregnancy related complications such as pre-eclampsia, GDM, obesity, uterus rupture, and among others (25). While women with advanced maternal age (AMA) are more prone to develop pregnancy-related complications. AMA is defined as age more than or equal to 35 years (26,27). The reference sample group also has normal BMI. BMI is an important variable that needs to be considered when establishing and/or verifying cut-off points for glucose. BMI is used to classify adiposity, and adiposity is known to be associated with glucose metabolism (28). Studies have shown that there is an association between maternal BMI and obesity with development of GDM and other pregnancy complications (29,30). Weight, weight gain, and BMI are modifiable risk factors of GDM development and should be monitored to prevent the condition (4,31). Thus, to prevent bias due to weight and BMI, this study included only pregnant women with normal pre-pregnancy BMI as part of the reference sample group. Similarly, BP was also considered in choosing members of the reference sample group. Only those with normal BP were included in this study. Normal BP for pregnant women according to the ACOG is below 120/80 mmHg. It is important to identify BP of pregnant women included in the reference sample group because studies have shown that high BP is a risk factor for GDM development (31,32).
Aside from the abovementioned demographic variables, this study also considered pre-analytical variables that may cause small incremental changes in glucose concentrations influencing GDM diagnosis. Pre-analytical factors like the use of tubes containing anti-glycolytic agents, immediate separation of cells and storage temperature as key roles in the levels of glucose concentrations are important things to consider. Glycolysis ex vivo is one of the major sources of uncertainties in glucose determinations. An average reduction of glucose concentration of 5–7% per hour can occur, especially when high leukocyte blood counts and high temperatures are present (33). It was also shown that glucose concentrations in unpreserved blood samples decreases rapidly at room temperature (34). Moreover, prolonged time elapsing between blood drawing to the centrifugation and separation of cell mass may negatively affect the detection of diabetes and elevate the rate of misdiagnosis (35). In one study for instance, OGTT was performed using a serum tube and centrifugation was not done until the last collection, thereby falsely decreasing the glucose values because of glycolysis ex vivo (36). This was supported by another study wherein they were able to show an increase in the prevalence of GDM from 11.6% to 20.6% when the protocol of immediate centrifugation within 10 minutes after venipuncture was followed. It was noted in that study that there is an increase in the fasting plasma glucose concentration by 0.24 mmol/L with the change in protocol contributed most to this increase in diagnosis rate (37). According to WHO and American National Academy of Clinical Biochemistry (NACB), the sample to be used must be venous plasma placed in ice-water slurry after collection and separation of cells must be within 30 minutes (36). In this present study, venous blood samples were collected, and placed in serum separator tubes. As presented in the methodology, specimens were allowed to clot, then blood samples were centrifuged for 15 minutes at 2,500 RPM. Serum was immediately separated from the red bloods cells, and utilized for glucose measurement. Following these procedures would prevent unnecessary effects of the identified pre-analytical variables.
Another potential source of variation in OGTT results is the AOG when OGTT is being performed. Performance of OGTT in early trimester of pregnancy indicates that a pregnant mother is at high risk to develop GDM. While performance of OGTT on the late trimester of pregnancy may indicate that the mother is at low risk to develop GDM (23,38). This risk assessment is performed by their obstetrician-gynecologists (39). Early or late performance of OGTT has advantages and disadvantages. On one hand, early testing, although may detect GDM early on and prevent potential complications, has a high probability of false negative results, and may only need re-testing later on when doctors observe abnormalities on the course of pregnancy (23,39). On the other hand, late testing may cause late diagnosis when complications of GDM are already present.
Although some studies mentioned about variability in glucose levels depending on the AOG (21,23,40), this present study was able to remove that source of variation. OGTT results of the reference sample group whose testing was performed on or before 28 weeks did not significantly differ from the OGTT results of those whose testing was done after 28 weeks of gestation (P=0.05). Similar results have been observed by previous studies as well. In one study, no significant difference on the OGTT results taken on the 16th week versus 36th week of gestation was observed (41). Moreover, in another study where prevalence of GDM was studied in different trimesters of pregnancies, they observed that glucose intolerance occurs in all trimesters of pregnancies; although, they recommended repeat OGTT up to 36 weeks of gestation or beyond to confirm negative GDM diagnosis (42).
Taking a closer look at Table 5, we can say that cut-off pointb is not a good set of cut-off points, with specificities and positive predictive value (PPV) below 60% in 1st and 2nd hour glucose cut-off points. We are now left with choosing between cut-off pointa or cut-off pointc. Although cut-off pointa and cut-off pointc are more or less the same, we can observe higher specificity and PPV for FBS alone in cut-off pointc, but higher specificity and PPV for both 1st hour and 2nd hour blood glucose in cut-off pointa. With this, we are recommending here the use of cut-off pointa: FBS >4.8; 1st hour blood glucose >7.5; and 2nd hour blood glucose >6.6. As with the number of threshold values that needs to be met to diagnose GDM, we recommend reaching at least one threshold value to consider the presence of GDM. The use of one threshold value of cut-off pointa provided good positive likelihood ratios of 10.2 for FBS alone, 7.11 for 1st hour serum glucose alone, and 8.82 for 2nd hour serum glucose alone (data not included in the table). More than 1 positive likelihood ratio and less than 0.1 negative likelihood ratio indicate a very useful tool to establish diagnosis and exclude a diagnosis. The use of two or more threshold values did not result to better diagnostic efficiency as shown in the table. After identifying the recommended cut-off points and the number of threshold values to meet to diagnose GDM, we look at the difference in terms of number of individuals diagnosed when using the standard criteria of IADPSG versus when using the recommended cut-off points, that is cut-off pointa with one, two or three threshold values to meet (Figure 4). The use of one threshold value of cut-off pointa allowed better GDM diagnosis, with prevalence higher than when using IADPSG criteria. The use of two or more threshold values provided stricter rules, which limits the diagnosis of the condition.
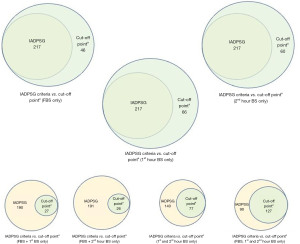
To summarize, the guidelines we utilized in deciding on which cut-off point to use and the number of threshold values to meet, we listed the following:
- High sensitivity (>90%);
- At least 80% specificity;
- Higher NPV than PPV but not too low PPV (at least 70%).
High sensitivity is a pre-requisite for a screening test. Studies mentioned that if a medical decision limit is being established for screening a certain condition, high sensitivity is desirable (15,43,44). The high sensitivity of the recommended medical decision limit or cut-off point and number of threshold value will allow an individual to be confident of not having GDM if the test is negative (15). Although we prioritize sensitivity over specificity, specificity obtained utilizing cut-off pointa is not low and is also acceptable and will allow an individual to be assured of having the condition when the test is positive (>80%) (15). Similarly, when deciding which is more desirable in terms of high PPV versus high NPV, we considered the condition we wanted to diagnose, which is GDM. Variety of circumstances were considered, namely, consequences of not being able to detect the condition (false negative), high-cost requirement in treating the condition (false positive), and effect of treatment to the mother and the fetus (false positive). High PPV over NPV would entail higher unnecessary cost and overtreatment. High NPV over PPV, on the other hand, will miss out patients with GDM for being monitored and treated (15). Because GDM poses serious complications to both the mother and the fetus, it is more necessary to avoid false negative decisions. A higher NPV over PPV is more acceptable to prevent misdiagnosis of GDM and thus, avoid adverse pregnancy outcomes.
Conclusions
With variability in glucose levels depending on population, we were able to establish in this study the cut-off points to interpret 75-g OGTT among Filipino pregnant women. We are proposing the use of these values: FBS >4.8 mmol/L; 1st hour blood glucose >7.5 mmol/L, and 2nd hour blood glucose >6.6 mmol/L. At least one of these three cut-off points should be met to diagnose GDM. The use of this cut-off points is comparable with the IADPSG criteria but more suitable among Filipino pregnant women. A limitation of our study is the incorporation of the correlation of maternal and perinatal outcomes when using our recommended cut-off points. This may be performed in future studies, together with further validation of the recommended cut-off points involving Filipino pregnant women in other areas of the Philippines.
Acknowledgments
The authors thank the Department of Science and Technology – Philippine Council for Health Research and Development (DOST – PCHRD).
Funding: This publication was part of the “Gestational Diabetes Mellitus Study” on potential identification of early biomarkers of the disease (Grant No. 18-0200) supported and funded by DOST-PCHRD. The funding organization had no role in the study design, data collection, data analysis, manuscript preparation and/or publication decisions.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-22-26/rc
Data Sharing Statement: Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-22-26/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-22-26/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by University of Santo Tomas Ethics Review Committee (No. E-2016-02-R3) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Schneider AJ. Some thoughts on normal, or standard, values in clinical medicine. Pediatrics 1960;26:973-84. [Crossref] [PubMed]
- Ceriotti F. Prerequisites for use of common reference intervals. Clin Biochem Rev 2007;28:115-21. [PubMed]
- Habibzadeh F, Habibzadeh P, Yadollahie M. On determining the most appropriate test cut-off value: the case of tests with continuous results. Biochem Med (Zagreb) 2016;26:297-307. [Crossref] [PubMed]
- Alejandro EU, Mamerto TP, Chung G, et al. Gestational Diabetes Mellitus: A Harbinger of the Vicious Cycle of Diabetes. Int J Mol Sci 2020;21:5003. [Crossref] [PubMed]
- O'Malley EG, Reynolds CME, O'Kelly R, et al. The diagnosis of gestational diabetes mellitus (GDM) using a 75 g oral glucose tolerance test: A prospective observational study. Diabetes Res Clin Pract 2020;163:108144. [Crossref] [PubMed]
- Hatem M, Anthony F, Hogston P, et al. Reference values for 75 g oral glucose tolerance test in pregnancy. Br Med J (Clin Res Ed) 1988;296:676-8. [Crossref] [PubMed]
- Moses RG, Moses M, Russell KG, et al. The 75-g glucose tolerance test in pregnancy: a reference range determined on a low-risk population and related to selected pregnancy outcomes. Diabetes Care 1998;21:1807-11. [Crossref] [PubMed]
- Tomkins M, Smith D. Should we continue to use the 75-g OGTT to diagnose diabetes? Ir J Med Sci 2020;189:525-7. [Crossref] [PubMed]
- Huhn EA, Rossi SW, Hoesli I, et al. Controversies in Screening and Diagnostic Criteria for Gestational Diabetes in Early and Late Pregnancy. Front Endocrinol (Lausanne) 2018;9:696. [Crossref] [PubMed]
- McIntyre HD, Colagiuri S, Roglic G, et al. Diagnosis of GDM: a suggested consensus. Best Pract Res Clin Obstet Gynaecol 2015;29:194-205. [Crossref] [PubMed]
- Pineda-Cortel MRB, Manalo MEM, Canivel RRC, et al. Screening and Diagnosis of Gestational Diabetes Mellitus Using 75-g Oral Glucose Tolerance Test Following the WHO, ADA, and IADPSG Criteria. Journal of Diabetes & Metabolism 2018; [Crossref]
- Behboudi-Gandevani S, Amiri M, Bidhendi Yarandi R, et al. The impact of diagnostic criteria for gestational diabetes on its prevalence: a systematic review and meta-analysis. Diabetol Metab Syndr 2019;11:11. [Crossref] [PubMed]
- Serafica-Hernandez SA, Espina-Tan C, Tremedal A, et al. Local versus International Criteria in Predicting Gestational Diabetes Mellitus-Related Pregnancy Outcomes. Philipp J Obstet Gynecol 2014;38:33-42.
- Shang M, Lin L. IADPSG criteria for diagnosing gestational diabetes mellitus and predicting adverse pregnancy outcomes. J Perinatol 2014;34:100-4. [Crossref] [PubMed]
- Bishop ML, Schoeff LE, Fody EP. Clinical Chemistry Principles, Techniques and Correlations. 7th ed: Philadelphia: Wolters Kluwer Health/Hippincott Williams & Wilkins; 2013.
- Ozarda Y. Establishing and using reference intervals. Turkish Journal of Biochemistry 2020;45:1-10. [Crossref]
- Bujang MA, Adnan TH. Requirements for Minimum Sample Size for Sensitivity and Specificity Analysis. J Clin Diagn Res 2016;10:YE01-6. [Crossref] [PubMed]
- Unal I. Defining an Optimal Cut-Point Value in ROC Analysis: An Alternative Approach. Comput Math Methods Med 2017;2017:3762651. [Crossref] [PubMed]
- Hajian-Tilaki K. Receiver Operating Characteristic (ROC) Curve Analysis for Medical Diagnostic Test Evaluation. Caspian J Intern Med 2013;4:627-35. [PubMed]
- Baduashvili A, Guyatt G, Evans AT. ROC Anatomy-Getting the Most Out of Your Diagnostic Test. J Gen Intern Med 2019;34:1892-8. [Crossref] [PubMed]
- Frøslie KF, Røislien J, Qvigstad E, et al. Shape information in repeated glucose curves during pregnancy provided significant physiological information for neonatal outcomes. PLoS One 2014;9:e90798. [Crossref] [PubMed]
- Iwama N, Sugiyama T, Metoki H, et al. Difference in the prevalence of gestational diabetes mellitus according to gestational age at 75-g oral glucose tolerance test in Japan: The Japan Assessment of Gestational Diabetes Mellitus Screening trial. J Diabetes Investig 2019;10:1576-85. [Crossref] [PubMed]
- Nakanishi S, Aoki S, Kasai J, et al. High probability of false-positive gestational diabetes mellitus diagnosis during early pregnancy. BMJ Open Diabetes Res Care 2020;8:e001234. [Crossref] [PubMed]
- Bogdanet D, O'Shea P, Lyons C, et al. The Oral Glucose Tolerance Test-Is It Time for a Change?-A Literature Review with an Emphasis on Pregnancy. J Clin Med 2020;9:3451. [Crossref] [PubMed]
- Bellieni C. The Best Age for Pregnancy and Undue Pressures. J Family Reprod Health 2016;10:104-7. [PubMed]
- Attali E, Yogev Y. The impact of advanced maternal age on pregnancy outcome. Best Pract Res Clin Obstet Gynaecol 2021;70:2-9. [Crossref] [PubMed]
- Biagioni EM, May LE, Broskey NT. The impact of advanced maternal age on pregnancy and offspring health: A mechanistic role for placental angiogenic growth mediators. Placenta 2021;106:15-21. [Crossref] [PubMed]
- Ng CD, Elliott MR, Riosmena F, et al. Beyond recent BMI: BMI exposure metrics and their relationship to health. SSM Popul Health 2020;11:100547. [Crossref] [PubMed]
- Teshome AA, Li Q, Garoma W, et al. Gestational diabetes mellitus, pre-pregnancy body mass index and gestational weight gain predicts fetal growth and neonatal outcomes. Clin Nutr ESPEN 2021;42:307-12. [Crossref] [PubMed]
- Badon SE, Dublin S, Nance N, et al. Gestational weight gain and adverse pregnancy outcomes by pre-pregnancy BMI category in women with chronic hypertension: A cohort study. Pregnancy Hypertens 2021;23:27-33. [Crossref] [PubMed]
- Hedderson MM, Ferrara A. High blood pressure before and during early pregnancy is associated with an increased risk of gestational diabetes mellitus. Diabetes Care 2008;31:2362-7. [Crossref] [PubMed]
- Li G, Huang W, Zhang L, et al. A prospective cohort study of early-pregnancy risk factors for gestational diabetes in polycystic ovarian syndrome. Diabetes Metab Res Rev 2018;34:e3003. [Crossref] [PubMed]
- Carta M, Giavarina D, Paternoster A, et al. Glucose meters: What's the laboratory reference glucose? J Med Biochem 2020;39:32-9. [PubMed]
- Coward SM, O'Neill FC, McAdam L, et al. Stabilization of Plasma Glucose: The Use of Newer Technology and Pragmatic Laboratory Practice. J Appl Lab Med 2019;3:1028-34. [Crossref] [PubMed]
- Codish S, Amichay D, Yitshak-Sade M, et al. Improvement of Blood Samples Preanalytic Management Alters the Clinical Results of Glucose Values: Population Study. J Diabetes Sci Technol 2020;14:284-9. [Crossref] [PubMed]
- García-Del-Pino I, Bauça JM, Gómez C, et al. Preanalytical issues related to routine and diagnostic glucose tests: Results from a survey in Spain. Biochem Med (Zagreb) 2020;30:010704. [PubMed]
- Potter JM, Hickman PE, Oakman C, et al. Strict Preanalytical Oral Glucose Tolerance Test Blood Sample Handling Is Essential for Diagnosing Gestational Diabetes Mellitus. Diabetes Care 2020;43:1438-41. [Crossref] [PubMed]
- Arendz IJ, Oomen PH, Wolthuis A, et al. Prevalentie van diabetes gravidarum bij risicozwangeren. Ned Tijdschr Geneeskd 2013;157:A5409. [PubMed]
- Rani PR, Begum J. Screening and Diagnosis of Gestational Diabetes Mellitus, Where Do We Stand. J Clin Diagn Res 2016;10:QE01-4. [Crossref] [PubMed]
- Katayama H, Tachibana D, Hamuro A, et al. Sustained Decrease of Early-Phase Insulin Secretion in Japanese Women with Gestational Diabetes Mellitus Who Developed Impaired Glucose Tolerance and Impaired Fasting Glucose Postpartum. Jpn Clin Med 2015;6:35-9. [Crossref] [PubMed]
- Siegmund T, Rad NT, Ritterath C, et al. Longitudinal changes in the continuous glucose profile measured by the CGMS in healthy pregnant women and determination of cut-off values. Eur J Obstet Gynecol Reprod Biol 2008;139:46-52. [Crossref] [PubMed]
- Seshiah V, Balaji V, Balaji MS, et al. Gestational diabetes mellitus manifests in all trimesters of pregnancy. Diabetes Res Clin Pract 2007;77:482-4. [Crossref] [PubMed]
- Trevethan R. Sensitivity, Specificity, and Predictive Values: Foundations, Pliabilities, and Pitfalls in Research and Practice. Front Public Health 2017;5:307. [Crossref] [PubMed]
- Burtis C, Ashwood E, Burns D. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. 5th ed, 2011. 13th December 2011. doi:
10.1093/clinchem/48.1.213a .10.1093/clinchem/48.1.213a
Cite this article as: Pineda-Cortel MRB, Suratos T, Mamerto TP. Cut-off points of 75-gram oral glucose tolerance test as a diagnostic test for gestational diabetes mellitus in pregnant Filipino population. J Lab Precis Med 2022;7:31.


