
Could body temperature be used as surrogate measure of mRNA-based vaccination efficacy in the general population?
Introduction
Predicting the humoral, cellular and especially clinical response to coronavirus disease 2019 (COVID-19) vaccination remains a central aspect for efficiently tackling the ongoing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic. Several lines of evidence attest that, like other types of vaccines, reactogenicity to the different COVID-19 vaccine formulations may be considerably attenuated by a kaleidoscope of demographical and clinical factors, as extensively summarized elsewhere (1), and encompassing characteristics such as older age, male sex, obesity, cancers, chronic impairment of liver and kidney function, pathological or pharmacological immunosuppression, as well as type, dosage and number of inoculations of the currently available COVID-19 vaccines.
Several current studies have been focusing on predicting the clinical response to vaccination by testing both immunological (i.e., anti-SARS-CoV-2 antibodies) (2) and cellular [i.e., interferon-gamma release assays (IGRAs)] (3,4) biomarkers, or a combination of both (5,6). Nonetheless, a strategy encompassing the assessment of humoral, cellular immunity or both is plagued by a number of drawbacks, including the cost for collecting the blood and performing the assays, the limited throughput and turnaround time of currently available IGRAs, the often unpredictable response of all serological and cellular assays versus new SARS-CoV-2 variants with highly mutated antigenic epitopes (7), as well as the long turnaround time (from 1 to several days) for reporting test results to the stakeholders.
In this puzzling scenario, characterized by insufficient and unequal COVID-19 vaccine global coverage (8), the availability of a “biological marker” that may help predicting vaccine efficacy, thus efficiently surrogating laboratory-based tests, may be seen as an extremely valuable resource for optimizing vaccine delivery to those who will need additional boosters, while concomitantly saving vaccine doses in those who instead may not really need them. To this end, the foremost question here and now is: “do we have such magic bullet”? Although it is inherently challenging to respond to this question, interesting evidence is emerging that fever, one very frequent and relatively specific post-vaccine clinical symptoms, may provide valuable clue on this matter.
Body temperature variation following mRNA-based COVID-19 vaccination
Although body temperature monitoring for screening SARS-CoV-2 infection carries a number of drawbacks (9), further amplified in vaccinated and/or those infected by the new Omicron lineages who may be frequently asymptomatic or only mildly symptomatic (10), a febrile response has been frequently recorded in subjects receiving mRNA-based COVID-19 vaccination, and the extent of such response has been consistently associated with humoral immunity in a number of recent studies in the general population (mostly encompassing healthcare workers), the most representative of which will be briefly summarized here.
Otani et al. carried out a cross-sectional including 338 healthcare workers who were administrated with two standard doses of BNT162b2 vaccine, asked to register relevant post-vaccine side effects with a self-administered questionnaire, and also had their anti-SARS-CoV-2 S IgG antibodies assayed 3 months after vaccination (11). The serum levels anti-SARS-CoV-2 antibodies were found to be nearly 50% higher [i.e., 5,132 vs. 3,515 arbitrary units (AU)/mL; P=0.002] in patients who experienced fever after completing the primary vaccination cycle compared to those who did not.
In a subsequent study, Koike et al. followed-up 378 healthcare workers up to 6 months after completing a primary vaccination cycle with the BNT162b2 vaccine, who were instructed to use a structured self-report questionnaire for reporting post-vaccination symptoms (12). The onset of fever following both the first and second vaccine doses was found to be positively associated with significantly higher total anti-SARS-CoV-2 spike antibodies levels measured 3 months after vaccination (i.e., between 23–24% higher), evidencing also a significant positive association between antibodies levels and body temperature (ρ=0.166; P=0.010).
Yamamoto et al. studied 88 members of a medical institution in Japan, who also underwent primary vaccination with the BNT162b2 vaccine, and were asked to record fever onset within four days post-vaccination with an online questionnaire (13). The serum levels of anti-SARS-CoV-2 S IgG measured between 7–74 days after completing vaccination were significantly higher in vaccine recipient reporting moderate (i.e., 38.0–38.4 ℃; +60%) or high (i.e., ≥38.5 ℃; +118%) body temperature compared to those without fever.
Very similar evidence emerged from a subsequent study conducted by Tani et al. (14), who followed up 335 hospital healthcare workers undergoing primary vaccination with the BNT162b2 vaccine, who were asked to self-annotate post-vaccination symptoms for up to 5 days. The values of anti-SARS-CoV-2 S IgG measured within 2 months post-vaccination (i.e., after the second dose) were found to be significantly higher in patients with modest elevation of body temperature (i.e., 37.0–37.9 ℃; 9,374 AU/mL) or frank fever (i.e., ≥38.5 ℃; 13,035 AU/mL) compared to those without body temperature elevation (i.e., <37.0 ℃; 7,186; P<0.001 for both comparisons). Accordingly, the extent of body temperature elevation after completing vaccination was found to be significantly correlated with the IgG antibodies levels (standardized beta coefficient, 0.301; P<0.001).
Kobashi et al. studied 231 healthcare workers who were subjected to a primary vaccination cycle with the BNT162b2 vaccine (15). The presence of fever (i.e., >37.5 ℃) after completing the primary vaccination cycle was found to be the strongest factor associated with anti-SARS-CoV-2 S IgG antibodies values measured 3 weeks post-vaccination (beta coefficient, 473.7; P<0.001).
Not only body temperature has been associated with the humoral response developing after primary COVID-19 vaccination, but a significant correlation has been demonstrated with anti-SARS-CoV-2 antibodies measured after vaccine boosters. Kawasuji and colleagues studied 565 healthcare workers who received a first booster of the BNT162b2 vaccine, who were asked to record the onset of post-vaccine symptoms with a dedicated questionnaire (16). The serum levels of anti-SARS-CoV-2 S total antibody levels measured 2 weeks after vaccine booster were found to be significantly higher in individuals with fever (i.e., ≥37.5 ℃) than in those without (>25,000 vs. ~20,000 AU/mL; P<0.001). In keeping with these findings, Matsumoto et al. enrolled 47 subjects among the university faculty staff, who received the third (booster) dose of the mRNA-1273 vaccine and were surveyed online for symptoms onset up to one-week post-vaccination (17). The serum levels of anti-SARS-CoV-2 S IgG and IgM, assayed up to 1 month after vaccination, displayed a much faster increase in those who developed fever (i.e., >37.5 ℃) than in those who did not. More specifically, the fixed-effect coefficient for the interaction term between fever and time (days) was 960 AU/mL.
Body temperature for screening vaccine immunogenicity
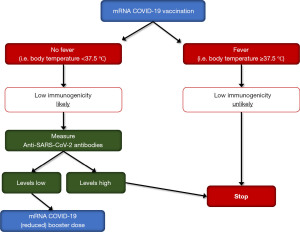
The results of all the aforementioned studies, summarized in Table 1, are highly suggestive of a significant relationship between increased body temperature and humoral response after mRNA-based COVID-19 vaccination. Although the paucity of studies addressing such relationships in recipients of other types of COVID-19 vaccines (e.g., adenoviral, inactivated, protein-based) precludes to generalize these observation, it seems reasonable to conclude that fever should be no longer considered only an adverse—almost undesirable—post-vaccination side effect, wherein its onset may actually reflect enhanced immunological response, which could be used for predicting vaccine immunogenicity in terms of anti-SARS-CoV-2 antibodies generation up to 3 months after mRNA-based COVID-19 vaccination. In a world with limited resources, considering that universal post-vaccination screening would be unfeasible for both organization and economical reasons, we suggest that body temperature monitoring post-COVID-19 vaccination may be used as a possible marker of vaccine humoral response and thus allowing to efficiency developing and validating specific algorithms, such as that tentatively provided in Figure 1. Briefly, in mRNA-based COVID-19 vaccine recipients who developed a febrile reaction (i.e., body temperature ≥37.5 ℃, which may reflect persistent immunogenic response to the antigen), low immunogenicity is unlikely and, thereby, no additional vaccine booster dose (in the immediate future) and/or no further testing may be needed. Contrarily, in mRNA-based COVID-19 vaccine recipients who did not report fever (i.e., body temperature <37.5 ℃), lower immunogenicity is more likely, such that laboratory assessment of anti-SARS-CoV-2 total or IgG antibodies may be warranted. In afebrile subjects who then display a sufficient antibody levels (defined according to method-specific cut-offs), no additional interventions may be needed, whilst an additional vaccine booster may be considered in those with low anti-SARS-CoV-2 antibodies titers, perhaps administering a reduced booster doses (i.e., half the standard dosage), which elicit comparable immunogenicity but lower serious side effects compared to the normal dosage (18).
Table 1
| Study | Study population | Vaccination | Antibodies (time of assessment) | Outcome |
|---|---|---|---|---|
| Otani et al., 2021 (11) | 338 healthcare workers | Primary vaccination with BNT162b2 | Anti-SARS-CoV-2 S IgG (3 months) | 50% higher in those with fever |
| Koike et al., 2022 (12) | 378 healthcare workers | Primary vaccination with BNT162b2 | Anti-SARS-CoV-2 S total (3 months) | 23–24% higher in those with fever |
| Yamamoto et al., 2022 (13) | 88 healthcare workers | Primary vaccination with BNT162b2 | Anti-SARS-CoV-2 S IgG (7–74 days) | 60–118% higher in those with fever |
| Tani et al., 2022 (14) | 335 healthcare workers | Primary vaccination with BNT162b2 | Anti-SARS-CoV-2 S IgG (2 months) | 30–81% higher in those with fever |
| Kobashi et al., 2022 (15) | 231 healthcare workers | Primary vaccination with BNT162b2 | Anti-SARS-CoV-2 S IgG (3 weeks) | Fever strongest predictor of humoral response |
| Kawasuji et al., 2022 (16) | 565 healthcare workers | BNT162b2 (first) booster | Anti-SARS-CoV-2 S total (2 weeks) | >25% higher in those with fever (unreported values) |
| Matsumoto et al., 2022 (17) | 47 university workers | mRNA-1273 (first) booster | Anti-SARS-CoV-2 S IgG and IgM (1 month) | Faster increase of antibodies levels in those with fever |
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-22-59/coif). GL serves as the Editor-in-Chief of Journal of Laboratory and Precision Medicine. The other authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lippi G, Henry BM, Plebani M. Optimizing effectiveness of COVID-19 vaccination: will laboratory stewardship play a role? Clin Chem Lab Med 2021;59:1885-8. [Crossref] [PubMed]
- Cromer D, Steain M, Reynaldi A, et al. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe 2022;3:e52-61. [Crossref] [PubMed]
- Jaganathan S, Stieber F, Rao SN, et al. Preliminary Evaluation of QuantiFERON SARS-CoV-2 and QIAreach Anti-SARS-CoV-2 Total Test in Recently Vaccinated Individuals. Infect Dis Ther 2021;10:2765-76. [Crossref] [PubMed]
- Krüttgen A, Klingel H, Haase G, et al. Evaluation of the QuantiFERON SARS-CoV-2 interferon-ɣ release assay in mRNA-1273 vaccinated health care workers. J Virol Methods 2021;298:114295. [Crossref] [PubMed]
- Vogrig M, Berger AE, Bourlet T, et al. Monitoring of Both Humoral and Cellular Immunities Could Early Predict COVID-19 Vaccine Efficacy Against the Different SARS-CoV2 Variants. J Clin Immunol 2022; Epub ahead of print. [Crossref] [PubMed]
- Desmecht S, Tashkeev A, El Moussaoui M, et al. Kinetics and Persistence of the Cellular and Humoral Immune Responses to BNT162b2 mRNA Vaccine in SARS-CoV-2-Naive and -Experienced Subjects: Impact of Booster Dose and Breakthrough Infections. Front Immunol 2022;13:863554. [Crossref] [PubMed]
- Lippi G, Adeli K, Plebani M. Commercial immunoassays for detection of anti-SARS-CoV-2 spike and RBD antibodies: urgent call for validation against new and highly mutated variants. Clin Chem Lab Med 2021; Epub ahead of print. [Crossref] [PubMed]
- Hassan F, London L, Gonsalves G. Unequal global vaccine coverage is at the heart of the current covid-19 crisis. BMJ 2021;375: [Crossref] [PubMed]
- Lippi G, Nocini R, Mattiuzzi C, et al. Is body temperature mass screening a reliable and safe option for preventing COVID-19 spread? Diagnosis (Berl) 2021;9:195-8. [Crossref] [PubMed]
- Blom K, Havervall S, Marking U, et al. Infection Rate of SARS-CoV-2 in Asymptomatic Healthcare Workers, Sweden, June 2022. Emerg Infect Dis 2022;28:2119-21. [Crossref] [PubMed]
- Otani J, Ohta R, Sano C. Association between Immunoglobulin G Levels and Adverse Effects Following Vaccination with the BNT162b2 Vaccine among Japanese Healthcare Workers. Vaccines (Basel) 2021;9:1149. [Crossref] [PubMed]
- Koike R, Sawahata M, Nakamura Y, et al. Systemic Adverse Effects Induced by the BNT162b2 Vaccine Are Associated with Higher Antibody Titers from 3 to 6 Months after Vaccination. Vaccines (Basel) 2022;10:451. [Crossref] [PubMed]
- Yamamoto S, Fukunaga A, Tanaka A, et al. Association between reactogenicity and SARS-CoV-2 antibodies after the second dose of the BNT162b2 COVID-19 vaccine. Vaccine 2022;40:1924-7. [Crossref] [PubMed]
- Tani N, Chong Y, Kurata Y, et al. Relation of fever intensity and antipyretic use with specific antibody response after two doses of the BNT162b2 mRNA vaccine. Vaccine 2022;40:2062-7. [Crossref] [PubMed]
- Kobashi Y, Shimazu Y, Kawamura T, et al. Factors associated with anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein antibody titer and neutralizing activity among healthcare workers following vaccination with the BNT162b2 vaccine. PLoS One 2022;17:e0269917. [Crossref] [PubMed]
- Kawasuji H, Morinaga Y, Tani H, et al. Effectiveness of the third dose of BNT162b2 vaccine on neutralizing Omicron variant in the Japanese population. J Infect Chemother 2022;28:1273-8. [Crossref] [PubMed]
- Matsumoto N, Kadowaki T, Matsuo R, et al. Association between fever and antibody titer trends after a third dose of the mRNA-1273 vaccine. J Epidemiol 2022; Epub ahead of print. [Crossref] [PubMed]
- Kanokudom S, Assawakosri S, Suntronwong N, et al. Comparison of the reactogenicity and immunogenicity of a reduced and standard booster dose of the mRNA COVID-19 vaccine in healthy adults after two doses of inactivated vaccine. Vaccine 2022;40:5657-63. [Crossref] [PubMed]
Cite this article as: Mattiuzzi C, Lippi G, Henry BM. Could body temperature be used as surrogate measure of mRNA-based vaccination efficacy in the general population? J Lab Precis Med 2022;7:33.


