
Measurement uncertainty in the VWF-ADAMTS13 axis: the challenges of adding a calculated measurand in the haemostasis laboratory
Introduction
Von Willebrand factor (VWF) is secreted from Wiebel-Palade bodies in the endothelium in response to inflammation or perturbation of the endothelial layer (1). VWF acts as a carrier to plasma Factor VIII and a mediator of platelet adhesion in regions of high shear stress. At rest, platelets and VWF do not interact. Platelet aggregation is regulated by interactions with the multimeric structure of the VWF molecule, its unravelling due to shear and subsequent cleavage.
VWF can be a complicated measurand to measure. Measurement of antigenic measures in plasma is the focus (2). Assays are validated for clinical and research use with antibodies directed to discrete epitopes of VWF in non-diseased plasma and patient plasma in verified von Willebrand Disease (VWD) (2). Detection methods range from change in absorbance due to a proportional colour change (3) to latex agglutination and chemiluminescence (4). Measurement of VWF activity is required to subtype VWD (5). Measurement focuses on the interaction of the VWF-A1 domain with GpIba on the platelet receptor GpIb/IX/V complex. In type 2 VWD and acquired VW syndrome (aVWS) a discrepancy between the amount of VWF and the functional activity may be observed (5). In the diagnostic setting, a variety of assays are used, in combination, to characterise the relationship. The diversity of tests available for characterisation of VWF in the research setting varies even more significantly.
ADAMTS13 (a-disintegrin and metalloproteinase with a thrombospondin type I motif, member 13), historically, referred to as VWF-cleaving protease, moderates the molecular distribution of the size of VWF multimers by cleavage at Tyr1605-Met1606 in the A2 domain of VWF (6). Severe deficiency of ADAMTS13, either due to a congenital deficiency or an immune mediated acquired deficiency cause a major imbalance between enzyme (ADAMTS13) and relatively normal levels of substrate (VWF-A2 domain). The effect is a persistence of ultra large (UL) VWF multimers (7). These multimers demonstrate hyperactive GpIba binding causing a fall in platelet count as a consequence of activation and clearance. Clots are formed in the small blood vessels, including those serving vital organs, resulting in a syndrome first described by Moschkowitz in 1924; thrombotic thrombocytopaenic purpura (TTP) (8). What is less clear is at what point the balance between ADAMTS13 and VWF shifts towards causing an excess of UL molecular weight multimers when a mildly reduced ADAMTS13 activity is observed in the presence of a markedly raised VWF.
Quantification of ADAMTS13 activity was historically very difficult and poorly standardised due to the need for substrate (VWF) unravelling that in the absence of shear forces in vitro had to be achieved chemically (9). The development and widespread availability of truncated peptides containing the VWF-A2 cleavage site brought significant improvements in laboratory standardisation. One such truncated peptide, VWF-73 ranges from residues 1596 to 1668 of the VWF-A2 domain and includes the Tyr1605-Met1606 cleavage site (10). Using this peptide, ADAMTS13 activity can be measured by interpolation from an in-run specific calibration curve that historically was constructed using a local plasma pool and latterly to calibration standards metrologically traceable to the WHO 1st international standard for ADAMTS13 (12/252) (11). As with VWF a range of different methods, using truncated substrates, are available. These include Enzyme Linked Immunosorbent Assay (ELISA) methods, Fluorescent Resonance Energy Transfer (FRET) (9) and recently a lateral flow type assay intended for rapid quantification (Technoclone, Austria) (12). Within the diagnostic laboratory, the ELISA and FRET methods have dominated and these themselves have evolved to faster throughput manufacturer methods such as the AcuStar chemiluminescent immunoassay (CIA) from Werfen (Bedford, USA) (13) and Technofluor Assay available on the Ceveron platform (Technoclone).
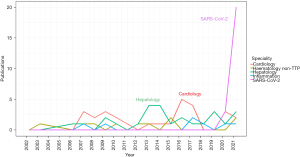
Historically the focus of ADAMTS13 measurement was on diagnosis and monitoring of TTP. An imbalance between VWF and ADAMTS13 much subtler than that seen in TTP has generated significant interest during the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic. This has added to an already considerable body of literature reporting VWF/ADAMTS13 imbalance in non-TTP clinical scenarios (see Figure 1). The balance is often represented by a ratio but inconsistently. For replication of results and translation to clinical practice it is clear standardisation is required using the same tools required of the ADAMTS13 and VWF assays individually.
Measurement uncertainty (MU) is “a non-negative parameter characterising the dispersion of quantity values being attributed to a measurand based on the information used” (14). Simply put, a measurement is only complete once MU is added to the estimated quantity of the measurand. This provides a measure of the quality of the result and allows complete interpretation and comparison of results, within and between patients—a property that should be seen as essential in all results generated by a clinical laboratory. Appropriate assessment of MU permits measurands to graduate from the research setting to clinical laboratory practice. The VWF-ADAMTS13 ratio is itself required to comply with this.
The aim of this review is to summarise the published literature on VWF/ADAMTS13 imbalance in pathophysiological disease. In doing so, and by considering the role of MU, we consider the challenges associated with interpretation of these studies and suggest what is required to standardise the derived result to maximise clinical utility.
Methods
Eligibility criteria
Any studies that were reported with the finding of an imbalance of VWF and ADAMTS13 as a major finding were included in the analysis.
Search strategy for identification of studies
In 2022, the author searched Medline, Ovid, PubMed, Google Scholar and Science Direct for studies reporting measurement of both VWF and ADAMTS13 and reporting results as a ratio. All results of any VWF assays were included, including VWF:Ag, VWF:RCo, unspecified “activity” and Collagen Binding Assay (CBA/VWF:CB). ADAMTS13 assays performed by FRET, CBA, ELISA and automated methods were all included. Boolean search was performed on each database using the search term: [(ADAMTS13) AND (von Willebrand factor)) AND (imbalance), (ADAMTS13) AND (von Willebrand factor)] AND (ratio) and derivatives of those. Studies reported prior to the search date of March 2022 with no limit to search start date were included. The earliest study reported was from 2002. The search was limited to the medical field (not including dentistry), studies reporting findings in human subjects, and articles available in the English language.
Study selection
All citations identified from the search were downloaded into Microsoft Excel (Microsoft Corporation, Redmond, WA, USA). The citations were organized and duplicates were identified and deleted. Unpublished articles were not considered in this review.
Data extraction
Study characteristics were extracted from each article based on the year of publication and clinical scenario reported. Findings were summarised as descriptions of overall study findings as well as specific reports of VWF-ADAMTS13 ratio described in each manuscript. The laboratory methods used for VWF and ADAMTS13 quantification were recorded for comparison. Methodologies used in the studies including reported units, traceability to higher order calibrations and performance characteristics were recorded when available. Data were analysed using Microsoft Excel 2016 (Microsoft Corporation, 2016) and the statistical programming environment R (R Core Team, Austria).
In studies where the VWF-ADAMTS13 ratio was not explicitly reported, a summary ratio was determined from raw data and/or figures available in the original manuscripts. From these results, studies were classified into whether they were reporting low, medium or high imbalances defined by the author as normal or reversed (0–1), low (2–5), medium (6–10) and high (>10). Care was taken to ensure reported units were correctly identified and for those where a classification into the above categories was not possible an additional category of “other ratio reported” was used.
VWF-ADAMTS13 imbalance associated with presenting condition
The influence of SARS-CoV-2 is noteworthy with regards to the number of publications citing VWF-ADAMTS13 ratios as a significant finding (Figure 1). Historically, reporting of VWF-ADAMTS13 ratio imbalance was inconsistent across disease presentations and this continued during the pandemic. However, it is clear that the absolute value of ratios reported across different disease presentation differs. Summary findings of all studies are presented in https://cdn.amegroups.cn/static/public/jlpm-22-48-1.xlsx.
Cardiology
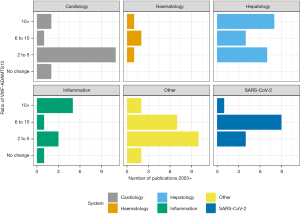
VWF/ADAMTS imbalance is reported across the full spectrum of cardiac disease probably not least due to well documented endothelial dysfunction and microvasculature perturbation (5), with both processes associated with ADAMTS13 and VWF levels and function. It is a feature of the studies collectively that although VWF-ADAMTS13 ratios are increased, they are mainly in the range of a 2–5-fold increase (Figure 2, https://cdn.amegroups.cn/static/public/jlpm-22-48-1.xlsx). Such increases are largely due to a markedly raised VWF and somewhat smaller reduction in ADAMTS13. Applications of findings range from prediction of severity of disease to identifying underlying pathophysiological mechanisms.
In a study of 80 patients with acute myocardial infarction, followed up immediately post event for 3–4 days and for the following 3 months; the VWF-ADAMTS13 ratio was increased by 50% (15). Following percutaneous coronary intervention post STEMI there was no correlation observed between VWF-ADAMTS13 ratio and infarct size, although interestingly VWF activity was higher and ADAMTS13 lower (resulting in a ratio around 2) in patients for whom an intramyocardial haemorrhage was seen (16). Two studies in atrial fibrillation (AF) (both performed using established commercial methods from American Diagnostica and Technoclone) found uncharacteristically high (>10) VWF-ADAMTS13 ratios and were performed by the same group (17,18). The hazard ratio of major adverse cardiac events was associated with a raised VWF-ADAMTS13 ratio and a reduced ADAMTS13 activity below the reference range (49.8%) had a hazard ratio of 1.833 [95% confidence interval (CI): 1.089–3.086; P=0.023]. No such association was observed for a raised VWF alone, demonstrating the added value in calculating the VWF-ADAMTS13 ratio.
Inflammation
Inflammation induces high cross talk between different regulators including VWF, neutrophil extracellular traps (NETs) and ADAMTS-13 (19). VWF increase along with an ADAMTS13 reduction are associated with NET activation. This is potentially mediated through a cocktail of different cytokines, as is often seen generally in inflammation but also in SARS-CoV-2, sepsis and thrombotic microangiopathy (TMA).
The release of UL VWF are induced by IL-6, IL-8, and TNF amongst others (19). In conjunction with the raised VWF UL VWF multimer levels. ADAMTS13 cleavage of these ULVWF multimers is also downregulated by IL-6 causing a double hit, persisting the ULVWF in the circulation for longer than usual. Peetermans reported VWF-ADAMTS13 ratios as high as 20 in 89 patients with staphylococcus aureus bacteraemia and VWF-ADAMTS13 ratio correlated with neutrophil count, C-reactive protein and D-Dimer (20). In critically ill patients where transfusions were required, the already activated endothelium becomes more activated and a consequent increase in VWF-ADAMTS13 ratio is observed of 11.6 (7.2–18.0) that persists for 24 hours after the transfusion to 12.8 (8.4–17.2) (21).
SARS-CoV-2
The presence of haematological, and haemostatic, laboratory abnormalities in SARS-CoV-2 was evident from the early stages of the SARS-CoV-2 pandemic (22). An extensive review by Favaloro, Henry and Lippi in 2021 highlights all studies up to that time that had investigated the association of reduced ADAMTS13, raised VWF by a number of different VWF assays, including VWF:RCo, VWF:GpIbR and VWF:Ab and SARS-CoV-2 (23). VWF levels are significantly raised in SARS-CoV-2. These are often 4–5-fold what would be considered normal (https://cdn.amegroups.cn/static/public/jlpm-22-48-1.xlsx). Equally, ADAMTS13 activity in SARS-CoV-2 patients is mildly reduced resulting in significantly imbalanced VWF/ADAMTS13 ratios (Figure 2). This leads to increased ULVWF multimers both tethered to the endothelium and also in the circulation leading to microthrombi formation (24). Raised VWF levels persist for at least 6 months post discharge (25).
In studies of SARS-CoV-2, VWF/ADAMTS13 ratio was always correlated to either clinical severity of disease or as a marker for diagnosis compared to a reference or control sample (https://cdn.amegroups.cn/static/public/jlpm-22-48-1.xlsx). This was applied in different ways including based on hospital location of the patient (24,26-28) extent of ventilator support required (29,30) or association with other clinical markers associated with SARS-CoV-2 (26,31,32).
Compared to cardiology, SARS-CoV-2 has a more marked increase in VWF-ADAMTS13 ratio across the studies reported, consistently in the range of 6 to 10 (Figure 2). The imbalance is driven by significantly increased VWF levels. In 75 patients with SARS-CoV-2, with mild to critical severity, the VWF-ADAMTS13 ratio was raised in the presence of what would be considered a largely normal ADAMTS13 activity of 67.8%±22.4% (33).
SARS-CoV-2 associated mortality correlates with VWF-ADAMTS13 ratio. In 53 patients in a single centre study a strong correlation was observed between ratio and mortality with non-survivors having a higher VWF-ADAMTS13 ratio of 4.94 (3.13–7.21) compared to survivors 3.18 (2.59–4.88) (34). A suggested mechanism for reduced ADAMTS13 is consumption without ruling out additional anti-ADAMTS13 proteolytic activity in SARS COV-2, under high inflammatory conditions (19). This may produce lower molecular weight versions (that are less catalytically active) than native ADAMTS13. Extent of ventilator support was also associated with degree of increase in VWF-ADAMTS13 ratio in a cross sectional study of 50 patients where a stepwise association between ventilator support requirements was seen (30). In a cross sectional study of 543 patients stratified by body mass index (BMI) and age, the increase in VWF-ADAMTS13 ratio was largest in older patients (>65 years) compared to those with a raised BMI (>30 kg/m2) (35).
Importantly, despite the markedly raised VWF-ADAMTS13 ratios reported throughout the literature, the prevalence of TMA is not altered in SARS COV-2. Rather it is suggested by Joly et al. that the VWF:ADAMTS13 ratio is a useful biomarker of endothelitis (increasing VWF levels) and consumption and/or inhibition of ADAMST13 (as seen in other inflammatory conditions) and does not account for the increased prevalence of thrombosis seen in SARS COV-2 (34). Lämmle et al. (36) in response to a study by Pascreau et al. (37) proposed a mechanism whereby SARS-CoV-2 virus infiltration of endothelial cells caused prolonged, sustained release of VWF from endothelium that is summarily cleaved by ADAMTS13 located within the area, that after a while becomes depleted and reflects the mildly reduced ADAMTS13 activities reported. This is a similar mechanism seen following desmopressin treatment (38) and in cases of severe sepsis (36) and surgery (39).
In acute kidney injury where acute reperfusion induces oxidative stress, human recombinant ADAMTS13 reduced this effect (40). Progression to severe SARS COV-2 and also severe acute kidney injury were predicted by VWF-ADAMTS13 ratio. A single unit change in the ratio is associated with a 20% decreased risk of AKI (41).
Hepatology and related conditions
Hepatology and inflammatory conditions showed the largest ratio increases (Figure 1). Hepatology showed significant VWF increase but a more substantial ADAMTS13 decrease than that seen in other disorders, most likely due to the decreased synthesis of ADAMTS13 in hepatic stellate cells in liver disease. In 676 patients with acute liver failure or acute liver injury the mean VWF:Ag levels were 448% [standard deviation (SD) 220.8], whereas the ADAMTS13 activity was 21.1% compared to the 80.9% of controls (42). The VWF-ADAMTS13 ratio was reported as high as 102.2±112.6 in severe acute hepatitis, compared to 10.6±11.6 in non-severe acute hepatitis (43), findings that were also shown in 24 patients with acute hepatitis (ratio 102) and liver cirrhosis (ratio 8.6) (44). Prolonged ratio increase was seen in 81 patients who underwent living donor liver transplantation up to a high of 40 over the 28-day period post-surgery (45). In patients with acute on chronic liver failure, VWF level and VWF-ADAMTS13 ratio predicted poor outcome compared to those who were discharged (46), as was the case in another 81 living liver donor patients who presented with a TMA-like syndrome with a significant difference between survivors and non-survivors in the VWF-ADAMTS13 ratio [10.7 (6.8–23.3) vs. 32.5 (15.6–46.7)] (47).
Other conditions
Imbalance of VWF:ADAMTS13 has been reported in trauma patients (48), acute liver failure (49) malaria (50) and during liver transplantation (51), See https://cdn.amegroups.cn/static/public/jlpm-22-48-1.xlsx. Additionally, the effect of systemic inflammation above advanced cirrhotic disease showed the ratio of VWF and ADAMTS13 to be predictive of clinical outcome (52) and more generally organ failure in association with severity of sepsis (53,54). Replenishment either by plasma or recombinant human ADAMTS13 (55) has been shown to be effective in treatment of trauma patients by reducing excessive ULVWF multimers to near normal levels.
Comparing pregnant women with preeclampsia, normotensive and non-pregnant women an increase in VWF-ADAMST13 ratio was observed that correlated with severity of symptoms experienced. The range increased from 2 in normotensive women to 4 in preeclampsia (56). VWF-ADAMTS13 balance is altered in diabetic patients and is related to estimated glomerular filtration rate (eGFR) (<60: 3.20±1.67 vs. >60: 2.34±1.39). Overall, diabetic patients have raised ratios of 2.58±1.52, most likely reflecting endothelial activation (57).
Applying the VWF-ADAMTS13 ratio in clinical practice
To be useful in routine clinical practice the application of a calculated parameter must consider a number of factors. These factors all contribute conceptually to the determination of the MU of the result. As such it is useful to appraise the published literature in the context of MU contributors to determine how, if at all, the results of published studies can inform practical clinical use.
Measurand definition and clinical validation of the assays in use
A measurand can equally be the result of a measurement process or can itself be an input into a further measurement process leading to a new measurand definition (58). In the case of the VWF-ADAMTS13 ratio, both VWF and ADAMTS13 are measurands—themselves with characterisable uncertainty—used as inputs to the calculated ratio. Defining the measurand, including types of samples used, is an essential first step in characterising MU. Sample types were largely from standard sodium citrate, although ethylene diamine tetra acetic acid (EDTA) was explicitly reported in at least one study for determination of VWF:Ag by in house ELISA (59), with a citrate being used for ADAMTS13 activity as EDTA cannot be used for that purpose (9).
Measurand definition—consistency of methods and description
An outline of all methods used—as reported in each study is provided in Table 1.
Table 1
| Body system classification | Total number of papers | Clinical Scenarios investigated for deranged VWF-ADAMTS13 ratio |
|---|---|---|
| Cardiology | 20 | Left atrial modelling in AF |
| Myocardial infarction | ||
| Neonatal congenital heart disease | ||
| Percutaneous coronary intervention | ||
| Peripheral artery disease | ||
| Unstable angina | ||
| Hepatology and related clinical disorders | 19 | Acute cholangitis |
| Acute liver failure | ||
| ACLF development and prognosis in liver cirrhosis | ||
| Alcoholic hepatitis | ||
| Hepatocellular carcinoma | ||
| Ischaemia reperfusion injury | ||
| Liver transplantation | ||
| Non-cirrhotic portal hypertension | ||
| Systemic inflammation and advanced cirrhotic disease | ||
| COVID-19 | 15 | SARS-CoV-2 |
| Haematology | 5 | beta thalassaemia |
| Desmopressin treatment | ||
| DVT/VTE | ||
| Sickle cell disease | ||
| Veno occlusive disease after stem cell transplantation | ||
| Immunology | 5 | Connective tissue diseases and antiphospholipid syndrome |
| HIV | ||
| Inflammation | ||
| Inflammatory bowel disease | ||
| Sepsis | 5 | Paediatric, pancreatitis, sepsis severity |
| Inflammation | 4 | Complement, sepsis, transfusion |
| Nephrology | 3 | Abdominal aortic aneurysm, chronic kidney disease |
| Neurology | 3 | Acute ischaemic brain injury, cerebrovascular disease, cerebral infarction |
| Obstetric | 3 | Pre-eclampsia, pregnancy and SARS-CoV-2 |
| Trauma | 3 | Acute kidney injury, trauma and replacement therapy |
| Respiratory | 3 | Advanced non-small cell lung cancer, community acquired pneumonia, transplantation |
| Paediatrics | 1 | Neonatal |
| Endocrinology | 1 | Diabetic nephropathy |
| Stroke | 1 | Cerebral artery thrombosis |
Publications are grouped according to body system classification, details of specific conditions within each classification are identified. VWF, von Willebrand factor; ADAMTS13, a disintegrin and metalloproteinase with thrombospondin type I motif, member 13; AF, atrial fibrillation; ACLF, acute on chronic liver failure; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; DVT, deep vein thrombosis; VTE, venous thromboembolism; HIV, human immunodeficiency virus.
VWF assays
The majority of reports quote the VWF:Ag as the measure of VWF although these are occasionally supplemented with additional VWF activity measurements (16,54,60,61). VWF multimer analysis is used rarely, and as it is a laborious and highly specialised method, the utility in routine clinical practice is questionable despite the additional information obtained by the relative distribution of VWF multimers.
The availability of commercial assays, be it in kit or automated form has influenced the ease with which a more standardised method can be used. Assays for VWF quantification were largely commercial with a mixture between commonly available antibodies sourced from Dako (21,29,39,42,43,49,51,53,62-72) and automated methods performed on high throughput automated coagulometers, either Siemens (16,57,73-75), STAGO (Liatest Assay) (75,76), Sysmex CS2000 (77), Werfen ACL-TOP (26,30,37,52,78-81), Behring (54) or Werfen AcuStar (27,30,32).
Commercial ELISAs were also available including the Asserachrom (Stago) (24,28,34,82-85), Hyphen (46,86), Immubind (45,87,88), Technoclone (48,89), American Diagnostica (17,18,56), READDS (90,91) and Abcam (35,92). In house methods are referenced throughout (33,59,93). Clearly comparing results across all of these methods, in the absence of a documented traceability chain for each should be approached with caution. For those using in-house methods, and in indeed for centres using commercially available methods, participation in proficiency testing schemes or in sample standardisation exercises is not reported so cannot be assessed.
There were apparent discipline specific method choices, no doubt associated with availability of technology in laboratory haematology compared to research groups. The majority of work published on SARS-CoV-2 were through highly automated coagulometers, the increased availability of which may have facilitated the rapid increase in publications from 2020 onwards. Often methods were reported as in-house (94) and without more detailed description assays using the rabbit anti-human polyclonal VWF antibody from Dako, could arguably be described as the same. These assays form the majority of the assays performed in the hepatology speciality for example. Some studies stated that the assays were performed elsewhere (95), and others reported they were performed by clinical laboratory established methods with no further details (96).
None of the assays, including the highly automated assays report linearity at the extreme levels being reported in each study. Additionally, clinical validations of assays use patient and control samples from known VWD or normal donors not suspected of having VWD. Patients with excessively raised UL-VWF multimers are not assessed in these studies. Methods are not validated at the extreme values reported throughout the literature and there is no accounting for how such high results were obtained—by dilution, extrapolation or any other method.
ADAMTS13 assays
ADAMTS13 methods used were more consistent albeit with the same variability of reporting. Commercial ELISA methods were amongst the most commonly used in particular from Kainos laboratories in Japan and Technoclone (Technozym) for both ADAMTS13 activity and antigen (17,18,24,33,35,52,57,74-76,89,90,91,97,98). Other less commonly used methods were American Diagnostica (15,81), Quantikine (37), Grifols DG-EIA (31), and Immunbind (56,83,86). Other ELISA methods were stated but with somewhat less detail (34,67,69,70,72,85,99).
Much of the early work was performed using the FRET method. FRET using VWF73 was the most common used (28-30,39,41,42,45,47,48,51,54,62,63,66,73,77,79,80,87,88,92,93,95), although studies have used others such as the VWF86 substrate (86). Older methods such as the collagen binding assay (CBA) based assay (38,46) or multimer based assays (71) are not frequently reported reflecting the advancements supported by the development of the truncated substrates.
In house methods again were used in early studies and are still present (21,43,49,54,55,68,100,101), although new to market rapid automated methods are beginning to present in published studies including the method on the AcuStar (Werfen, Bedford, USA) (20,26,27,32).
It is of note that some of the methods stated as in house or as general ELISA methods, particularly in the early years of VWF-ADAMTS13 investigation have gone on to be more standardised and have contributed to commercial method development. About twice as many studies combined commercial VWF and ADAMTS13 assays than a commercial method with an in house method.
Measurand definition assay selectivity and the sample matrix
Assay selectivity determines the ability of any measuring system to quantify each measurand independent of other biological analytes within the sample. Using assays in the presence of severe clinical disease, completely different from that seen in assay validation or verification puts selectivity claims into doubt. It is entirely likely, and more importantly untested, that there are interfering substances influencing final results. For example, the steric blockade of PF4 binding to VWF has important implications physiologically, by inhibiting access to the cleavage site on VWF, but also analytically, if, as is assumed, the binding of PF4 to VWF-73 truncated substrate is also affected by the presence of PF4. As such, consideration of how to interpret such results in unfamiliar clinical situations should be given. However, with the magnitude of changes reported in the studies reviewed here, such subtle effects may be insignificant to the VWF-ADAMTS13 ratio specifically. ADAMTS13 is negatively correlated with CRP (37,52) during systemic inflammation, and cholesterol in chronic kidney disease (95). Reduced kidney function is associated with both low ADAMTS13 and VWF-ADAMTS13 ratio (100).
Inflammatory cytokines, including IL-6 and IL-8, are negatively correlated with ADAMTS13 activity (69). IL-6 degrades ADAMTS13 as well as inhibiting ADAMTS13 synthesis (102), along with other proinflammatory mediators such as TNF-alpha. TSP-1 levels raised in inflammation interact with VWF (103,104), and in particular the A2 domain that causes in vitro issues with ADAMTS13 quantification by binding to the VWF73 truncated substrate (105). Additionally, protease released from neutrophils have been shown to inactivate ADAMTS13 activity (106). Oxidation of methionine residues (of which the cleavage site Met1606 of VWF-A2 is one) reduces ADAMTS13 cleavage of the VWF73 substrate also (105). Oxidation may be caused by reactive oxidation species including hydrogen peroxide, hypochlorous acid released by human neutrophil peptides (also known as alpha defensins) from neutrophil extracellular traps during inflammation (105). Extracellular haemoglobin (ECHb) binds to circulating VWF multimers via the A2-domain and significantly inhibits ADAMTS13 cleavage in patients with sickle cell disease (107). The severity of clinical disease represented in the publications studied demonstrate that some of these commonly occurring variations would be expected to impact on final reported results. It was not common practice to investigate this, nor acknowledge the impact this may have made.
Metrological traceability of the measurement result
Traceability through calibration
Both VWF and ADAMTS13 are calibrated assays, requiring construction of a standard curve to interpolate results. Only 14% of VWF assays and 11% of ADAMTS13 activity assays reported had calibration methods explicitly quoted (see Table 1). However, the use of commercial kits—particularly self-contained ELISA methods often utilise kit calibrators, the nature of their calibrators being specific to the method, and traceable based on information provided in kit inserts so the apparent low numbers do not accurately reflect compliance. The same can be said of assays performed on highly automated coagulometers that provide calibration plasmas traceable to the appropriate international standard (30) thereby permitting reporting results in the international unit, although this was not universally followed even for these assays (37). The majority of those studies that did report their source of calibration employed a local normal pooled plasma source as a standard and reported against this pool as 100% (39,42,49,51,63,97). Before the advent of widespread international standards this practice was commonplace for haemostasis measurements. The 6th international FVIII/VWF standard (07/316, NIBSC, Potters Bar, UK) with assigned values (108) was quoted in a small number of studies (55) as being used for their VWF assay as were earlier versions (93) but this was not the norm. Of course, availability of international standards has not been widespread for some measurands for the period of these studies so this must be considered, but implemented in future practice as best practice. As previously stated proteolytic cleavage of ADAMTS13 during inflammatory responses has been reported. This produces a different, but still catalytically active form. Traceability to the 12/252 international standard as routine ADAMTS13 assays are has not been confirmed.
Traceability to reported units
The nature of the reported ratio remains inconsistent with combinations of ADAMTS13 activity or antigen used as either the denominator or numerator for calculation of the ratio. Invariably this changes not only the absolute value, and interpretation, of the ratio, but additionally the units reported for each measurement. Conversion of one ratio to the other requires some elementary mathematical manipulation and for this review results where relevant have been standardised to the more commonly reported VWF-ADAMTS13 ratio. Intuitively in the scenarios discussed here it makes logical sense to report VWF-ADAMTS13 ratio in this manner as the change in the ratio is influenced by small to medium reductions in ADAMTS13 compared to large increases to VWF level and therefore representing the ratio as increasing with the VWF being the most significant driver is appropriate. However, standardisation of calculated parameters acknowledging whether representation should be as substrate/enzyme or enzyme/substrate has not been satisfactorily determined.
In practice, a ratio would ideally have no unitary assignment as the division of the two parameters used for the calculation should be normalised to the same unit. Inconsistent units can be handled by sensitivity coefficients using the law of propagation of uncertainty.
Metrological traceability of the equipment and methods
Clinically significant differences have been documented between different types of VWF activity assays (109). This leads to, potentially, different diagnosis in up to 20% of VWD patients. This is in the context of a patient population selected for investigation. How those differences change when non-validated patient populations, such as those with severe inflammation, or SARS COV-2 diagnosis is unknown. The type of collagen used will result in differential sensitivity for ULVW multimers with type III collagen being more sensitive (3).
Imprecision, bias and bias correction
A central contributor to MU estimation in the clinical laboratory is long term imprecision. Generally, this is derived from the performance of internal quality control (IQC), but may in the initial phase use manufacturer specifications quoted in instructions for use. The extent to which these metrics were available in published studies is shown in Table 1. The purpose of IQC is to confirm linearity between calibration points to ensure linearity of method in points interpolated between calibration dilutions. IQC both for these assays, and as a mechanism for determining imprecision are not in the regions where patient results are being reported. As such an issue arises where imprecision of the method—at the levels reported is not assured.
The impact this has is variable depending on methods used. For highly automated assays, including rapid VWF and ADAMTS13 assays now in use, IQC is an appropriate method of characterising imprecision. However, for so called one off experiments such as ELISA methods repeatability measures of IQC may be less informative. Instead IQC checks alignment of the procedure respective to traceable values assigned to calibrators—at levels distinct from the levels reported in these studies. This may be thought of as a representation of bias from calibration, itself to be assessed for significance, corrected if possible and the uncertainty of the correction applied. This is not routinely considered in the assays shown here, or indeed many haemostasis assays where this must apply. Guidance is required to determine the best way to incorporate IQC from one off methods into uncertainty assessments. The provided imprecision expectations from manufacturers can be used as a surrogate, with the caveat that often the methods used for determining these performance characteristics are unknown and therefore imprecision should be represented as a type B uncertainty.
Calculated parameters and propagation of uncertainty
The Law of Propagation of Uncertainty (110) states how input measurands pass their uncertainty onto a calculated measurand. Propagation of uncertainty is routine when no correlation exists between the input measurands of the calculated measurement equation. However, if correlation exists, it must be accounted for. From the studies reviewed here, in the quoted clinical scenarios, VWF:Ag and ADAMTS13 activity and antigen are negatively correlated. Although reported in only a few studies, negative correlation ranged from −0.302 in patients with unstable or exertional angina (82) to −0.4846 in SARS-CoV-2, although Peetermans (20) reported a non-significant negative correlation of −0.1684. The consequence of not accounting for such correlation will be an underestimation of the combined uncertainty and a direct impact on interpretation of clinical results.
Performance specifications
Expected performance specifications, whether measures of precision or even a reference range, or those that were achieved are by no means standard practice for reporting of laboratory based methods throughout. This was reflected in the studies reviewed here; 12.0% of studies reviewed here presented specifications in one form or another, and these were almost exclusively in recognition of manufacturer quoted specifications. Although not a requirement for interpretation of results, when derived quantities are being quoted, these are very helpful to the reader. Additionally, sensitivity, specificity and associated performance characteristics such as positive and negative predictive value require an understanding of the impact of MU on clinical cut-off points that determine classification.
Condition specific reference ranges
Throughout all publications presented here there is a clear trend of raised VWF and reduced ADAMTS13 associated with a wide range of clinical conditions. What is clear is that these results are abnormal with reference to so called normal or disease free subjects. For adequate interpretation of the results, and for monitoring over time, a representation of what may be expected in “stable” disease may be of interpretive benefit. Clearly, the number and sample sizes within such studies do not, currently, inform expected biological variation in disease states, and this is a common shortfall of much of the biological variation data for haemostasis parameters. The difficulties with this are compounded when combining results of two measurands both of which are being used outside their validated clinical diagnostic uses.
Reference change values and the VWF/ADAMTS13 axis
Unsurprisingly a high number of studies report a correlation between stage of disease and VWF-ADAMTS13 ratio. The impact of this is that if the ratio is to be a useful marker for disease progression, an understanding of how results change in a given patient, in that clinical scenario, is required so that a potential intervention could be considered. Traditionally, the reference change value, incorporating MU (or often solely analytical imprecision) and biological variation incorporates this understanding. There is no acknowledgement of MU in any of the studies reported here, and therefore, in the absence of biological variation data, or standardised performance specifications there is no standardised way to demonstrate a significant difference between serial results in patients from these published data.
Conclusions
The wide range of thrombotic manifestations, and often normal, or near normal ADAMTS13 activity levels, in conjunction with high VWF levels confirm that there are many different mediators of VWF function, only some of which have been identified. It remains to be seen what the other, as yet, unidentified measurands are, and whether they themselves interact with ADAMTS13 and VWF to further complicate the interactions. However, the association of the VWF-ADAMTS13 ratio in non-haematological disorders remains useful as evidenced by its widespread adoption in the research field. Attempts have even been made to correlate the ratio with established clinical scoring systems [PRISM score (94) and APACHE II score (69)]. Reporting bias may contribute to only 5 studies reported negative findings (55,66,74,75,90) for VWF-ADAMTS13 ratio.
Introduction of rapid, automated methods on readily available coagulation platforms such as the AcuStar and Ceveron mean that a quick turnaround result is possible. This has seen a step change in clinical ordering practices (author’s experience). We are seeing a more diverse set of patients being investigated for ADAMTS13 deficiency, for the purposes of TTP exclusion, rather than the familiar positive predictive value we are accustomed to. It remains unclear whether the enthusiasm for investigations of the VWF-ADAMTS13 axis that has been ignited during the SARS-CoV-2 pandemic persists.
Clearly, the extent to which VWF-ADAMTS13 ratios are investigated throughout the literature highlight the perceived importance of this physiological axis. The range of otherwise unrelated disorders that quote alterations in the baseline ratio mean that the interaction of VWF with ADAMTS13, and no doubt many other players mean there is a future where the measurement of this axis is going to be of substantial clinical importance for diagnosis, monitoring and treatment outcomes of patients. The need for application of the ratio in clinical outcome studies is clear. In order to establish the VWF-ADAMTS13 ratio as a meaningful biological derived parameter the time has come to apply the same statistical and quality assurance based rigour to this derived parameter as is the case for individual measurands, including VWF and ADAMTS13 individually. This includes determining true baseline assessments of these parameters and their ratios in real patients and also determining whether monitoring over time is a practical way to monitor disease progression. For this to be successful MU and biological variation need to be considered at the forefront of priorities. It is through the behaviours and variation in these performance specifications that a robust assessment of changes within and between patients can be made, across time, between centres and across methodologies. Knowledge and appreciation of the performance of each of the methods, their applicability to each clinical situation and the impact of pre analytical variables including sample quality and matrix effects means that there is a lot of work to do to standardise interpretation of the VWF-ADAMTS13 ratio. However, once those steps have been taken, a diverse range of fields within medicine see the conceptual benefits of monitoring this new biomarker, and in the haemostasis laboratory it is down to us to make sure that we provide the best results possible to support that.
Finally, the data presented here are dominated by studies that have reviewed the balance between a raised VWF and reduced ADAMTS13. Care should also be taken to consider the converse relationship whereby a reduced VWF relative to normal, perhaps due to raised ADAMTS13 may induce an acquired von Willebrand syndrome (VWS) and may result in bleeding (111). Additionally, raised ADAMTS13 levels relative to normal have been reported in venous thromboembolism (112), and while this is not a common finding, the initial acute elevation in ADAMTS13 reported may serve as a compensatory mechanism for managing the ULVWF released from endothelium acutely, until such a time as the ADAMTS13 becomes consumed and levels decrease. Not discussed in this review is the utility of functional assays of haemostasis including viscoelastometry for use in these patient cohorts. This was not investigated in any of the publications reviewed. Taken separately, viscoelastic testing does not add additional benefit to those assays routinely used for assessment of ADAMTS13 or VWF so it is reasonable to expect that no additional benefit would be seen with viscoelastic testing although we await data to assess this.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-22-48/prf
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-22-48/coif). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wagner DD. Cell biology of von Willebrand factor. Annu Rev Cell Biol 1990;6:217-46. [Crossref] [PubMed]
- Favaloro EJ. Classification of von Willebrand disease in the context of modern contemporary von Willebrand factor testing methodologies. Res Pract Thromb Haemost 2020;4:952-7. [Crossref] [PubMed]
- Favaloro EJ. Navigating the Myriad of von Willebrand Factor Assays. Hamostaseologie 2020;40:431-42. [Crossref] [PubMed]
- Bodó I, Eikenboom J, Montgomery R, et al. Platelet-dependent von Willebrand factor activity. Nomenclature and methodology: communication from the SSC of the ISTH. J Thromb Haemost 2015;13:1345-50. [Crossref] [PubMed]
- DiGiandomenico S, Christopherson PA, Haberichter SL, et al. Laboratory variability in the diagnosis of type 2 VWD variants. J Thromb Haemost 2021;19:131-8. [Crossref] [PubMed]
- Petri A, Kim HJ, Xu Y, et al. Crystal structure and substrate-induced activation of ADAMTS13. Nat Commun 2019;10:3781. [Crossref] [PubMed]
- Subhan M, Scully M. Advances in the management of TTP. Blood Rev 2022;55:100945. [Crossref] [PubMed]
- Moschkowitz E. Hyaline thrombosis of the terminal arterioles and capillaries: a hitherto undescribed dis- ease. Proc NY Pathol Soc 1924;24:21-4.
- Mackie I, Mancini I, Muia J, et al. International Council for Standardization in Haematology (ICSH) recommendations for laboratory measurement of ADAMTS13. Int J Lab Hematol 2020;42:685-96. [Crossref] [PubMed]
- Kokame K, Nobe Y, Kokubo Y, et al. FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol 2005;129:93-100. [Crossref] [PubMed]
- Hubbard AR, Heath AB, Kremer Hovinga JA, et al. Establishment of the WHO 1st International Standard ADAMTS13, plasma (12/252): communication from the SSC of the ISTH. J Thromb Haemost 2015;13:1151-3. [Crossref] [PubMed]
- Moore GW, Meijer D, Griffiths M, et al. A multi-center evaluation of TECHNOSCREEN(®) ADAMTS-13 activity assay as a screening tool for detecting deficiency of ADAMTS-13. J Thromb Haemost 2020;18:1686-94. [Crossref] [PubMed]
- Favaloro EJ, Mohammed S, Chapman K, et al. A multicenter laboratory assessment of a new automated chemiluminescent assay for ADAMTS13 activity. J Thromb Haemost 2021;19:417-28. [Crossref] [PubMed]
- International vocabulary of metrology – Basic and general concepts and associated Terms (VIM). JCGM 2012.
- Al-Masri AA, Habib SS, Hersi A, et al. Effect of Acute Myocardial Infarction on a Disintegrin and Metalloprotease with Thrombospondin Motif 13 and Von Willebrand Factor and Their Relationship with Markers of Inflammation. Int J Vasc Med 2020;2020:4981092. [Crossref] [PubMed]
- Eerenberg ES, Teunissen PF, van den Born BJ, et al. The role of ADAMTS13 in acute myocardial infarction: cause or consequence? Cardiovasc Res 2016;111:194-203. [Crossref] [PubMed]
- Freynhofer MK, Bruno V, Jarai R, et al. Levels of von Willebrand factor and ADAMTS13 determine clinical outcome after cardioversion for atrial fibrillation. Thromb Haemost 2011;105:435-43. [Crossref] [PubMed]
- Freynhofer MK, Gruber SC, Bruno V, et al. Prognostic value of plasma von Willebrand factor and its cleaving protease ADAMTS13 in patients with atrial fibrillation. Int J Cardiol 2013;168:317-25. [Crossref] [PubMed]
- Yang J, Wu Z, Long Q, et al. Insights Into Immunothrombosis: The Interplay Among Neutrophil Extracellular Trap, von Willebrand Factor, and ADAMTS13. Front Immunol 2020;11:610696. [Crossref] [PubMed]
- Peetermans M, Meyers S, Liesenborghs L, et al. Von Willebrand factor and ADAMTS13 impact on the outcome of Staphylococcus aureus sepsis. J Thromb Haemost 2020;18:722-31. [Crossref] [PubMed]
- van Manen L, van Hezel ME, Boshuizen M, et al. Effect of red blood cell transfusion on inflammation, endothelial cell activation and coagulation in the critically ill. Vox Sang 2022;117:64-70. [Crossref] [PubMed]
- Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18:844-7. [Crossref] [PubMed]
- Favaloro EJ, Henry BM, Lippi G. Increased VWF and Decreased ADAMTS-13 in COVID-19: Creating a Milieu for (Micro)Thrombosis. Semin Thromb Hemost 2021;47:400-18. [Crossref] [PubMed]
- Philippe A, Gendron N, Bory O, et al. Von Willebrand factor collagen-binding capacity predicts in-hospital mortality in COVID-19 patients: insight from VWF/ADAMTS13 ratio imbalance. Angiogenesis 2021;24:407-11. [Crossref] [PubMed]
- Li H, Wu Q, Qin Z, et al. Serum levels of laminin and von Willebrand factor in COVID-19 survivors 6 months after discharge. Int J Infect Dis 2022;115:134-41. [Crossref] [PubMed]
- Dushianthan A, Abdul N, Dmochowski J, et al. Predictive Role of Haematological Determinants on Outcomes of Critically Ill COVID-19 Patients Admitted to Intensive Care Unit. Cureus 2021;13:e16764. [Crossref] [PubMed]
- Marco A, Marco P. Von Willebrand factor and ADAMTS13 activity as clinical severity markers in patients with COVID-19. J Thromb Thrombolysis 2021;52:497-503. [Crossref] [PubMed]
- Delrue M, Siguret V, Neuwirth M, et al. von Willebrand factor/ADAMTS13 axis and venous thromboembolism in moderate-to-severe COVID-19 patients. Br J Haematol 2021;192:1097-100. [Crossref] [PubMed]
- von Meijenfeldt FA, Havervall S, Adelmeijer J, et al. Prothrombotic changes in patients with COVID-19 are associated with disease severity and mortality. Res Pract Thromb Haemost 2020;5:132-41. [Crossref] [PubMed]
- Mancini I, Baronciani L, Artoni A, et al. The ADAMTS13-von Willebrand factor axis in COVID-19 patients. J Thromb Haemost 2021;19:513-21. [Crossref] [PubMed]
- Martín-Rojas RM, Chasco-Ganuza M, Casanova-Prieto S, et al. A mild deficiency of ADAMTS13 is associated with severity in COVID-19: comparison of the coagulation profile in critically and noncritically ill patients. Blood Coagul Fibrinolysis 2021;32:458-67. [Crossref] [PubMed]
- Rodríguez Rodríguez M, Castro Quismondo N, Zafra Torres D, et al. Increased von Willebrand factor antigen and low ADAMTS13 activity are related to poor prognosis in covid-19 patients. Int J Lab Hematol 2021;43:O152-5. [Crossref] [PubMed]
- Doevelaar AAN, Bachmann M, Hölzer B, et al. von Willebrand Factor Multimer Formation Contributes to Immunothrombosis in Coronavirus Disease 2019. Crit Care Med 2021;49:e512-20. [Crossref] [PubMed]
- Joly BS, Darmon M, Dekimpe C, et al. Imbalance of von Willebrand factor and ADAMTS13 axis is rather a biomarker of strong inflammation and endothelial damage than a cause of thrombotic process in critically ill COVID-19 patients. J Thromb Haemost 2021;19:2193-8. [Crossref] [PubMed]
- Thangaraju K, Katneni U, Akpan IJ, et al. The Impact of Age and BMI on the VWF/ADAMTS13 Axis and Simultaneous Thrombin and Plasmin Generation in Hospitalized COVID-19 Patients. Front Med (Lausanne) 2022;8:817305. [Crossref] [PubMed]
- Lämmle B, Rossmann H. Invited commentary to: ADAMTS13 deficiency is associated with abnormal distribution of von Willebrand factor multimers in patients with COVID-19 by Tiffany Pascreau et al. Letter to the Editors-in-Chief, Thrombosis Research. Thromb Res 2021;204:141-2. [Crossref] [PubMed]
- Pascreau T, Zia-Chahabi S, Zuber B, et al. ADAMTS 13 deficiency is associated with abnormal distribution of von Willebrand factor multimers in patients with COVID-19. Thromb Res 2021;204:138-40. [Crossref] [PubMed]
- Reiter RA, Knöbl P, Varadi K, et al. Changes in von Willebrand factor-cleaving protease (ADAMTS13) activity after infusion of desmopressin. Blood 2003;101:946-8. [Crossref] [PubMed]
- Hugenholtz GC, Ruitenbeek K, Adelmeijer J, et al. Development of a Hyperactive Primary Hemostatic System During Off-Pump Lung Transplantation Resulting From an Unbalance Between von Willebrand Factor and Its Cleaving Protease ADAMTS13. Am J Transplant 2015;15:1958-66. [Crossref] [PubMed]
- Zhou S, Guo J, Liao X, et al. rhADAMTS13 reduces oxidative stress by cleaving VWF in ischaemia/reperfusion-induced acute kidney injury. Acta Physiol (Oxf) 2022;234:e13778. [Crossref] [PubMed]
- Henry BM, Benoit SW, de Oliveira MHS, et al. ADAMTS13 activity to von Willebrand factor antigen ratio predicts acute kidney injury in patients with COVID-19: Evidence of SARS-CoV-2 induced secondary thrombotic microangiopathy. Int J Lab Hematol 2021;43:129-36. [Crossref] [PubMed]
- Driever EG, Stravitz RT, Zhang J, et al. VWF/ADAMTS13 Imbalance, But Not Global Coagulation or Fibrinolysis, Is Associated With Outcome and Bleeding in Acute Liver Failure. Hepatology 2021;73:1882-91. [Crossref] [PubMed]
- Ishikawa M, Uemura M, Matsuyama T, et al. Potential role of enhanced cytokinemia and plasma inhibitor on the decreased activity of plasma ADAMTS13 in patients with alcoholic hepatitis: relationship to endotoxemia. Alcohol Clin Exp Res 2010;34:S25-33. [Crossref] [PubMed]
- Matsuyama T, Uemura M, Ishikawa M, et al. Increased von Willebrand factor over decreased ADAMTS13 activity may contribute to the development of liver disturbance and multiorgan failure in patients with alcoholic hepatitis. Alcohol Clin Exp Res 2007;31:S27-35. [Crossref] [PubMed]
- Kobayashi T, Wada H, Usui M, et al. Decreased ADAMTS13 levels in patients after living donor liver transplantation. Thromb Res 2009;124:541-5. [Crossref] [PubMed]
- Prasanna KS, Goel A, Amirtharaj GJ, et al. Plasma von Willebrand factor levels predict in-hospital survival in patients with acute-on-chronic liver failure. Indian J Gastroenterol 2016;35:432-40. [Crossref] [PubMed]
- Takahashi N, Wada H, Usui M, et al. Behavior of ADAMTS13 and Von Willebrand factor levels in patients after living donor liver transplantation. Thromb Res 2013;131:225-9. [Crossref] [PubMed]
- Dyer MR, Plautz WE, Ragni MV, et al. Traumatic injury results in prolonged circulation of ultralarge von Willebrand factor and a reduction in ADAMTS13 activity. Transfusion 2020;60:1308-18. [Crossref] [PubMed]
- Hugenholtz GC, Adelmeijer J, Meijers JC, et al. An unbalance between von Willebrand factor and ADAMTS13 in acute liver failure: implications for hemostasis and clinical outcome. Hepatology 2013;58:752-61. [Crossref] [PubMed]
- Schwameis M, Schörgenhofer C, Assinger A, et al. VWF excess and ADAMTS13 deficiency: a unifying pathomechanism linking inflammation to thrombosis in DIC, malaria, and TTP. Thromb Haemost 2015;113:708-18. [Crossref] [PubMed]
- Pereboom IT, Adelmeijer J, van Leeuwen Y, et al. Development of a severe von Willebrand factor/ADAMTS13 dysbalance during orthotopic liver transplantation. Am J Transplant 2009;9:1189-96. [Crossref] [PubMed]
- Reuken PA, Kussmann A, Kiehntopf M, et al. Imbalance of von Willebrand factor and its cleaving protease ADAMTS13 during systemic inflammation superimposed on advanced cirrhosis. Liver Int 2015;35:37-45. [Crossref] [PubMed]
- Fukushima H, Nishio K, Asai H, et al. Ratio of von Willebrand factor propeptide to ADAMTS13 is associated with severity of sepsis. Shock 2013;39:409-14. [Crossref] [PubMed]
- Kremer Hovinga JA, Zeerleder S, Kessler P, et al. ADAMTS-13, von Willebrand factor and related parameters in severe sepsis and septic shock. J Thromb Haemost 2007;5:2284-90. [Crossref] [PubMed]
- Kleinveld DJB, Simons DDG, Dekimpe C, et al. Plasma and rhADAMTS13 reduce trauma-induced organ failure by restoring the ADAMTS13-VWF axis. Blood Adv 2021;5:3478-91. [Crossref] [PubMed]
- Alpoim PN, Gomes KB, Godoi LC, et al. ADAMTS13, FVIII, von Willebrand factor, ABO blood group assessment in preeclampsia. Clin Chim Acta 2011;412:2162-6. [Crossref] [PubMed]
- Prochazka V, Jonszta T, Czerny D, et al. The Role of von Willebrand Factor, ADAMTS13, and Cerebral Artery Thrombus Composition in Patient Outcome Following Mechanical Thrombectomy for Acute Ischemic Stroke. Med Sci Monit 2018;24:3929-45. [Crossref] [PubMed]
- Cox M, O’Hagan A. Meaningful expression of uncertainty in measurement. Accred Qual Assur 2022;27:19-37. [Crossref]
- Farkas P, Csuka D, Mikes B, et al. Complement activation, inflammation and relative ADAMTS13 deficiency in secondary thrombotic microangiopathies. Immunobiology 2017;222:119-27. [Crossref] [PubMed]
- Reiter RA, Varadi K, Turecek PL, et al. Changes in ADAMTS13 (von-Willebrand-factor-cleaving protease) activity after induced release of von Willebrand factor during acute systemic inflammation. Thromb Haemost 2005;93:554-8. [Crossref] [PubMed]
- Qu L, Jiang M, Qiu W, et al. Assessment of the Diagnostic Value of Plasma Levels, Activities, and Their Ratios of von Willebrand Factor and ADAMTS13 in Patients with Cerebral Infarction. Clin Appl Thromb Hemost 2016;22:252-9. [Crossref] [PubMed]
- Murphy SJX, Lim ST, Hickey F, et al. von Willebrand Factor Antigen, von Willebrand Factor Propeptide, and ADAMTS13 in Carotid Stenosis and Their Relationship with Cerebral Microemboli. Thromb Haemost 2021;121:86-97. [Crossref] [PubMed]
- Molvarec A, Rigó J Jr, Bõze T, et al. Increased plasma von Willebrand factor antigen levels but normal von Willebrand factor cleaving protease (ADAMTS13) activity in preeclampsia. Thromb Haemost 2009;101:305-11. [Crossref] [PubMed]
- Graham SM, Chen J, Le J, et al. Von Willebrand Factor Adhesive Activity and ADAMTS13 Protease Activity in HIV-1-Infected Men. Int J Med Sci 2019;16:276-84. [Crossref] [PubMed]
- Möller C, Schutte AE, Smith W, et al. Von Willebrand factor, its cleaving protease (ADAMTS13), and inflammation in young adults: The African-PREDICT study. Cytokine 2020;136:155265. [Crossref] [PubMed]
- Sedaghat S, de Vries PS, Boender J, et al. von Willebrand Factor, ADAMTS13 Activity, and Decline in Kidney Function: A Population-Based Cohort Study. Am J Kidney Dis 2016;68:726-32. [Crossref] [PubMed]
- Innami Y, Katori N, Mori K, et al. Increased prothrombotic property as a risk factor of acute kidney injury after surgical repair of abdominal aortic aneurysm: a prospective observational study. J Intensive Care 2014;2:46. [Crossref] [PubMed]
- Matsumoto M, Kawa K, Uemura M, et al. Prophylactic fresh frozen plasma may prevent development of hepatic VOD after stem cell transplantation via ADAMTS13-mediated restoration of von Willebrand factor plasma levels. Bone Marrow Transplant 2007;40:251-9. [Crossref] [PubMed]
- Morioka C, Uemura M, Matsuyama T, et al. Plasma ADAMTS13 activity parallels the APACHE II score, reflecting an early prognostic indicator for patients with severe acute pancreatitis. Scand J Gastroenterol 2008;43:1387-96. [Crossref] [PubMed]
- Takaya H, Namisaki T, Shimozato N, et al. ADAMTS13 and von Willebrand factor are useful biomarkers for sorafenib treatment efficiency in patients with hepatocellular carcinoma. World J Gastrointest Oncol 2019;11:424-35. [Crossref] [PubMed]
- Ko S, Chisuwa H, Matsumoto M, et al. Relevance of ADAMTS13 to liver transplantation and surgery. World J Hepatol 2015;7:1772-81. [Crossref] [PubMed]
- Yoshikawa T, Nomi T, Sakai K, et al. Ischaemia-reperfusion injury with Pringle’s maneuver induces unusually large von Willebrand factor multimers after hepatectomy. Thromb Res 2019;183:20-7. [Crossref] [PubMed]
- Papadakis DD, Politou M, Kompoti M, et al. Immunostimulation and Coagulopathy in COVID-19 Compared to Patients With H1N1 Pneumonia or Bacterial Sepsis. In Vivo 2022;36:954-60. [Crossref] [PubMed]
- Tscharre M, Tentzeris I, Vogel B, et al. Von Willebrand Factor and ADAMTS13 and long-term outcomes in patients undergoing percutaneous coronary intervention. Thromb Res 2020;196:31-7. [Crossref] [PubMed]
- Green D, Tian L, Greenland P, et al. Association of the von Willebrand Factor-ADAMTS13 Ratio With Incident Cardiovascular Events in Patients With Peripheral Arterial Disease. Clin Appl Thromb Hemost 2017;23:807-13. [Crossref] [PubMed]
- Cibor D, Owczarek D, Butenas S, et al. Levels and activities of von Willebrand factor and metalloproteinase with thrombospondin type-1 motif, number 13 in inflammatory bowel diseases. World J Gastroenterol 2017;23:4796-805. [Crossref] [PubMed]
- Taylor A, Vendramin C, Singh D, et al. von Willebrand factor/ADAMTS13 ratio at presentation of acute ischemic brain injury is predictive of outcome. Blood Adv 2020;4:398-407. [Crossref] [PubMed]
- Martín-Rojas RM, Chasco-Ganuza M, Casanova-Prieto S, et al. A mild deficiency of ADAMTS13 is associated with severity in COVID-19: comparison of the coagulation profile in critically and noncritically ill patients. Blood Coagul Fibrinolysis 2021;32:458-67. [Crossref] [PubMed]
- Ward SE, Fogarty H, Karampini E, et al. ADAMTS13 regulation of VWF multimer distribution in severe COVID-19. J Thromb Haemost 2021;19:1914-21. [Crossref] [PubMed]
- Pagliari MT, Boscarino M, Cairo A, et al. ADAMTS13 activity, high VWF and FVIII levels in the pathogenesis of deep vein thrombosis. Thromb Res 2021;197:132-7. [Crossref] [PubMed]
- Ma WH, Sheng L, Gong HP, et al. The application of vWF/ADAMTS13 in essential hypertension. Int J Clin Exp Med 2014;7:5636-42. [PubMed]
- Fuchigami S, Kaikita K, Soejima K, et al. Changes in plasma von Willebrand factor-cleaving protease (ADAMTS13) levels in patients with unstable angina. Thromb Res 2008;122:618-23. [Crossref] [PubMed]
- Warlo EMK, Pettersen AR, Arnesen H, et al. vWF/ADAMTS13 is associated with on-aspirin residual platelet reactivity and clinical outcome in patients with stable coronary artery disease. Thromb J 2017;15:28. [Crossref] [PubMed]
- Uemura T, Kaikita K, Yamabe H, et al. Changes in plasma von Willebrand factor and ADAMTS13 levels associated with left atrial remodeling in atrial fibrillation. Thromb Res 2009;124:28-32. [Crossref] [PubMed]
- Matsukawa M, Kaikita K, Soejima K, et al. Serial changes in von Willebrand factor-cleaving protease (ADAMTS13) and prognosis after acute myocardial infarction. Am J Cardiol 2007;100:758-63. [Crossref] [PubMed]
- Goel A, Alagammai PL, Nair SC, et al. ADAMTS13 deficiency, despite well-compensated liver functions in patients with noncirrhotic portal hypertension. Indian J Gastroenterol 2014;33:355-63. [Crossref] [PubMed]
- Ladeira VS, Barbosa AR, Oliveira MM, et al. ADAMTS-13-VWF axis in sickle cell disease patients. Ann Hematol 2021;100:375-82. [Crossref] [PubMed]
- Nobuoka Y, Wada H, Mizuno S, et al. Erratum to: Prolonged thrombocytopenia after living donor liver transplantation is a strong prognostic predictor irrespective of history of splenectomy: the significance of ADAMTS13 and graft function. Int J Hematol 2016;103:725-9. [Crossref] [PubMed]
- Plautz WE, Haldeman SH, Dyer MR, et al. Reduced cleavage of von illebrand factor by ADAMTS13 is associated with microangiopathic acute kidney injury following trauma. Blood Coagul Fibrinolysis 2022;33:14-24. [Crossref] [PubMed]
- Lee JM, Siddique J, Kim HC, et al. Hemostatic Markers and Long-Term Risk of Intracerebral Hemorrhage in Postmenopausal Women. J Stroke Cerebrovasc Dis 2016;25:1639-43. [Crossref] [PubMed]
- Hamed AA, Darwish YW, El-Sayed MH. ADAMTS13 Levels in Young Patients With β-Thalassemia Major: Relation to Hepatitis C Virus Infection, Liver Cirrhosis, and Iron Overload. Clin Appl Thromb Hemost 2015;21:527-32. [Crossref] [PubMed]
- Hunt R, Hoffman CM, Emani S, et al. Elevated preoperative von Willebrand factor is associated with perioperative thrombosis in infants and neonates with congenital heart disease. J Thromb Haemost 2017;15:2306-16. [Crossref] [PubMed]
- Gombos T, Makó V, Cervenak L, et al. Levels of von Willebrand factor antigen and von Willebrand factor cleaving protease (ADAMTS13) activity predict clinical events in chronic heart failure. Thromb Haemost 2009;102:573-80. [Crossref] [PubMed]
- Bongers TN, de Bruijne EL, Dippel DW, et al. Lower levels of ADAMTS13 are associated with cardiovascular disease in young patients. Atherosclerosis 2009;207:250-4. [Crossref] [PubMed]
- Shen L, Lu G, Dong N, et al. Von Willebrand factor, ADAMTS13 activity, TNF-α and their relationships in patients with chronic kidney disease. Exp Ther Med 2012;3:530-4. [Crossref] [PubMed]
- Tsai HM, Sarode R, Downes KA. Ultralarge von Willebrand factor multimers and normal ADAMTS13 activity in the umbilical cord blood. Thromb Res 2002;108:121-5. [Crossref] [PubMed]
- Turecek PL, Peck RC, Rangarajan S, et al. Recombinant ADAMTS13 reduces abnormally up-regulated von Willebrand factor in plasma from patients with severe COVID-19. Thromb Res 2021;201:100-12. [Crossref] [PubMed]
- Grandone E, Vimercati A, Sorrentino F, et al. Obstetric outcomes in pregnant COVID-19 women: the imbalance of von Willebrand factor and ADAMTS13 axis. BMC Pregnancy Childbirth 2022;22:142. [Crossref] [PubMed]
- Mobayen G, Dhutia A, Clarke C, et al. Severe COVID-19 is associated with endothelial activation and abnormal glycosylation of von Willebrand factor in patients undergoing hemodialysis. Res Pract Thromb Haemost 2021;5:e12582. [Crossref] [PubMed]
- Taniguchi S, Hashiguchi T, Ono T, et al. Association between reduced ADAMTS13 and diabetic nephropathy. Thromb Res 2010;125:e310-6. [Crossref] [PubMed]
- Denorme F, Kraft P, Pareyn I, et al. Reduced ADAMTS13 levels in patients with acute and chronic cerebrovascular disease. PloS One 2017;12:e0179258. [Crossref] [PubMed]
- Claus RA, Bockmeyer CL, Budde U, et al. Variations in the ratio between von Willebrand factor and its cleaving protease during systemic inflammation and association with severity and prognosis of organ failure. Thromb Haemost 2009;101:239-47. [Crossref] [PubMed]
- Bernardo A, Ball C, Nolasco L, et al. Effects of inflammatory cytokines on the release and cleavage of the endothelial cell-derived ultralarge von Willebrand factor multimers under flow. Blood 2004;104:100-6. [Crossref] [PubMed]
- Bonnefoy A, Daenens K, Feys HB, et al. Thrombospondin-1 controls vascular platelet recruitment and thrombus adherence in mice by protecting (sub)endothelial VWF from cleavage by ADAMTS13. Blood 2006;107:955-64. [Crossref] [PubMed]
- Chen J, Chung DW. Inflammation, von Willebrand factor, and ADAMTS13. Blood 2018;132:141-7. [Crossref] [PubMed]
- Mannucci PM, Capoferri C, Canciani MT. Plasma levels of von Willebrand factor regulate ADAMTS-13, its major cleaving protease. Br J Haematol 2004;126:213-8. [Crossref] [PubMed]
- Zhou Z, Behymer M, Guchhait P. Role of extracellular hemoglobin in thrombosis and vascular occlusion in patients with sickle cell anemia. Anemia 2011;2011:918916. [Crossref] [PubMed]
- Hubbard AR, Hamill M, Beeharry M, et al. Value assignment of the WHO 6th International Standard for blood coagulation factor VIII and von Willebrand factor in plasma (07/316). J Thromb Haemost 2011;9:2100-2. [Crossref] [PubMed]
- Boender J, Eikenboom J, van der Bom JG, et al. Clinically relevant differences between assays for von Willebrand factor activity. J Thromb Haemost 2018;16:2413-24. [Crossref] [PubMed]
- JCGM 100: Evaluation of measurement data – Guide to the expression of uncertainty of measurement. 2008.
- Horiuchi H, Doman T, Kokame K, et al. Acquired von Willebrand Syndrome Associated with Cardiovascular Diseases. J Atheroscler Thromb 2019;26:303-14. [Crossref] [PubMed]
- Mazetto BM, Orsi FL, Barnabé A, et al. Increased ADAMTS13 activity in patients with venous thromboembolism. Thromb Res 2012;130:889-93. [Crossref] [PubMed]
Cite this article as: MacDonald S. Measurement uncertainty in the VWF-ADAMTS13 axis: the challenges of adding a calculated measurand in the haemostasis laboratory. J Lab Precis Med 2023;8:5.


