
De Ritis ratio and cardiovascular disease: evidence and underlying mechanisms
Introduction
Aspartate aminotransferase [AST; Enzyme Commission (EC) 2.6.1.1] to alanine aminotransferase (ALT; EC 2.6.1.2) ratio was proposed in 1957 by De Ritis, Coltorti and Giusti (thereafter as, De Ritis ratio) as an enzymatic test for acute viral hepatitis (1). In the following years, De Ritis ratio found limited clinical use mostly as an indicator of alcohol-related liver disease (2) and a noninvasive marker of liver fibrosis (3,4). Although De Ritis ratio is simple to compute from readily available components (aminotransferase measurements), it is a highly complex biochemical metric that incorporates large amounts of difficult-to-interpret information. The concept that circulating aminotransferases and De Ritis ratio are merely markers of liver disease (or injury) is partially correct. Since transaminase reactions play a key role in metabolism, changes in circulating levels of aminotransferases and consequently the De Ritis ratio may mirror metabolic alterations or morbid conditions not necessarily related to liver disease. In recent years, the interest in the De Ritis ratio has resurrected and an array of studies have shown an association of De Ritis ratio with poor prognosis across a wide range of diseases including diabetes mellitus, cancer, diseases characterized by multiorgan failure, cardiovascular disease (CVD), stroke and corona virus disease (COVID)-19 (5-18). The literature on the association of De Ritis ratio with CVD has not been reviewed before. In this review, we tried to summarize the current knowledge on the association between De Ritis ratio and cardiometabolic risk and CVD. The existing medical literature in English language was assessed without restriction with respect to time or type of the study. The association of De Ritis ratio with hepatic disease or cancer was not covered.
Biology of aminotransferases
Aminotransferases are omnipresent enzymes that catalyze reversible transfer of amino group (-NH2) from amino acids to alpha-keto acids playing a fundamental role in the metabolism. The transamination reaction was discovered in muscle tissue in 1937 by Braunstein and Kritzmann (19). Aminotransferases are pyridoxal-5’-phosphate-dependent enzymes. Pyridoxal-5’-phosphate is a vitamin B6 derivative that participates directly in catalysis. Although the majority of structural amino acids (except for lysine, threonine, proline and hydroxyproline) undergo transamination, two aminotransferases—AST and ALT—are mostly active and abundant in cells. The ALT and AST-catalyzed reactions represent important metabolic links between amino acid and carbohydrate metabolism (20). AST and ALT measurements are routinely used to diagnose liver disease/injury, monitor therapy and disease progression and assess the prognosis of patients with liver disease. In addition, abnormal activities of ALT and AST are found in many extrahepatic diseases. Aminotransferase elevations in metabolic diseases like nonalcoholic fatty liver disease (NAFLD) may signify basic metabolic abnormalities at the amino acid and Krebs cycle levels (21). The structure, tissue distribution and metabolic functions of aminotransferases have been recently reviewed (22,23). The most fundamental metabolic functions of aminotransferases include: creation of balanced amounts of amino acids according to metabolic needs by reversible transfer of amino group between amino acids and alpha-keto acids; catabolism of amino acids providing substrates for citric acid cycle (and energy) or gluconeogenesis; maintenance of nicotinamide adenine dinucleotide (NAD+/NADH) ratio in cells via AST participation in the malate/aspartate shuttle across the inner mitochondrial membrane; ammonia transport and increased availability of glutamate and aspartate to cells (22,23).
AST is composed of two genetically and immunologically distinct isoenzymes: cytoplasmic AST (cAST or GOT1) and mitochondrial AST (mAST or GOT2) (24). The isoenzymes catalyze the same reaction, share a sequence homology of ~45% and appear to have evolved from a common ancestral gene (via gene duplication) (25). The gene for cytosolic AST is localized on chromosome 10 at the interface of bands q241-q251. Mitochondrial AST is characterized by a multigene family located on chromosomes 12 (p131-p132), 16 (q21), and 1 (p32-p33 and q25-q31) (26). However, only the gene located on chromosome 16 is functional whereas other genes are pseudogenes with unknown functions (26). Human ALT exists in two catalytically active isoforms: ALT1 and ALT2. There is also a third isoform (ALT2_2) with no enzymatic activity (27). The genes for human ALT (GPT1 and GPT2) are located on chromosomes 8 (band 8q24.3.) and 16 (band 16q12.1.), respectively (28,29). Human GPT1 is expressed in liver, kidney, intestine, myocardium, skeletal muscle, colon, pancreas, spleen and lung (30). ALT2 gene is expressed in skeletal muscle, brain, heart and white adipose tissue (30). ALT1 and ALT2 contribute to ALT activity in circulation but ALT1 is mainly responsible for basal ALT activity in human plasma (31). Studies in isolated organelles have shown that ALT1 is located in cytosol and endoplasmatic reticulum of hepatocytes but not in mitochondria whereas ALT2 is located in mitochondria and endoplasmatic reticulum in skeletal muscle cells (27). ALT2 contributes to circulating ALT levels, in conditions such as acute myocardial infarction or obesity (31) Regulation of ALT expression is unclear, but high protein intake, fasting, cortisol, glucagon, epinephrine and norepinephrine (mostly glyconeogenic stimuli) appear to induce ALT expression in rat liver (32,33). Some evidence suggests that ALT2 expression is regulated by androgens through activation of promoter androgen response element(s) (34). Regulation of expression of AST genes remains unknown, but expression of hepatic cytoplasmic AST appears to be under hormonal control. Glucocorticoid hormones induce AST gene expression at transcriptional level and the effect is potentiated by cyclic AMP and inhibited by insulin (35). Intracellular ALT expression is induced by peroxisome proliferator-activated receptor (PPAR) agonists (fenofibrate) and this mechanism might contribute to increased ALT activity in serum (36). The PPAR-alpha agonist fenofibrate (in mice) (37) and PPARγ agonist rosiglitazone (in adipocytes) (38) increase synthesis and release of cytoplasmic ALT and AST. The PPAR-alpha agonist AZD4619 induced only the human, but not the rat ALT1 gene promoter in a dose-dependent relationship (39). ALT and AST are highly heritable (20) with a heritability of approximately 0.5 in twin studies (40,41). Furthermore, a recent genome-wide association study suggested that AST and ALT are coheritable with a coheritability of 0.67 (42). This means that gene combinations favor elevation (or decrease) of expression of both enzymes and variants with high expression of one enzyme and low expression of the other are very rare.
The content and localization in cells and tissue distribution of aminotransferases are important factors underlying the levels of aminotransferases in serum. In the liver—the organ with highest levels of both aminotransferases—ALT is predominantly found in cytoplasm (80%) whereas AST is predominantly found in mitochondria. Depending on the degree of stress (and damage) on hepatocytes, the cytoplasmic fraction or mitochondrial fraction may be released from hepatocytes. Mild cell damage releases enzymes from the cytoplasm (soluble fraction) only, whereas severe necrotic lesions release enzymes from cytoplasm and mitochondria (43). This aspect is important for the understanding of the association between De Ritis ratio and liver diseases. Tissue distribution of ALT and AST differs widely. AST has a higher activity than ALT in all tissues. Expressed as a ratio to serum, AST/ALT activities are, 7,100/2,850 in liver, 7,800/450 in myocardium, 4,500/1,200 in kidney, 5,000/300 in skeletal muscle, 2,500/50 in brain, 1,400/100 in pancreas, 700/60 in spleen, 500/35 in lungs, 300/60 in intestine and 40/7 in red blood cells (2,44,45). The main source of ALT is liver whereas AST has multiple sources. The tissue distribution of aminotransferases allows assuming that pathological processes involving liver increase both ALT and AST whereas pathological processes in extra-hepatic tissue mostly increase AST. The level of tissue activity and the size of the organ may influence the level of aminotransferases in serum. Although ALT activity in skeletal muscle is approximately 10 times lower than in liver, skeletal muscle with a mass of 30 to 33 kg in adults is the main reservoir of AST in terms of quantity and a major source of enzyme release in skeletal muscle disease. AST activity and expression are higher in hepatocytes of periportal than perivenous zone of hepatic lobules (46,47). The periportal zone is nearest to blood supply, receives most oxygenated blood (48) and is the zone of hepatic lobules in which, gluconeogenesis, beta-oxidation of fatty acids and degradation of amino acids take place (49). Tissue distribution of AST and ALT is shown in Figure 1.
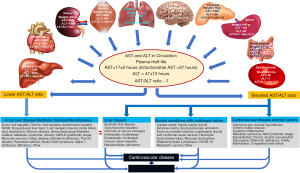
AST and ALT activity levels in serum are influenced by multiple factors. The underlying mechanisms of ALT release from the cells are unclear but may involve cellular leakage or cytoplasmic budding or blebbing into extracellular space and circulation. ALT1 accounts for most ALT in circulation (31). The current ALT assay measures combined ALT1 and ALT2 activity. ALT has a plasma half-life of 47±10 h, which is longer than plasma half-life of AST (17±5 h) (50). Elevation of AST in serum is explained by at least five mechanisms: cellular apoptosis (in the setting of physiological cellular renewal or augmented apoptotic stimuli); direct tissue damage (plasma membrane damage with protein leakage or cell necrosis caused by various noxious agents); plasma membrane blebs budding off from the cell membranes releasing cytoplasmic content; increased AST gene expression and macroenzymes (macroAST)—high-molecular-weight compounds that are formed by polymerization or association of AST with other serum proteins (typically with immunoglobulins) (51). Chronic alcohol use is particularly responsible for elevation of mitochondrial AST in serum. Elevated level of mitochondrial fraction of AST in serum indicates chronic rather than acute alcohol intake (52) and advanced alcohol-related liver disease rather than heavy drinking (53). Large organs with high AST and ALT activity contribute mostly to elevation of AST and ALT in serum (Figure 1). Aminotransferases are cleared from circulation mostly via hepatic uptake by sinusoidal liver cells (54,55). The kinetics of aminotransferase uptake is important because a decrease in hepatic function due to various diseases may prolong the hepatic clearance of aminotransferases leading to higher levels of these enzymes in serum. ALT in serum shows circadian variation being up to 45% higher in the afternoon hours than in morning hours and a 10–30% day-to-day variability (56,57). Muscular exercise (58), fast food-based hyper-alimentation combined with a sedentary lifestyle (59) sleep deprivation (60) or even eating quickly (61) and hospital admission (62) have been reported to influence (increase) aminotransferase levels. The hepatic and extrahepatic diseases associated with elevations of AST and ALT have recently been reviewed (63,64). ALT activity is higher in men than women possibly related to sex-specific hormonal differences (65). ALT activity is lower in patients on dialysis (66) and higher in overweight/obese subjects and patients with diabetes mellitus (67). ALT activity declines with weight loss but weight change appears not to alter AST activity (68). ALT levels decline with age in men and women, independent of metabolic traits, alcohol use, and other markers of hepatic function, and ALT may be considered a biomarker of aging (69). Aminotransferase levels appear to differ according to ethnicity. AST levels appear to be 15% higher in African American males compared with Caucasians (70) and Mexican Americans have a higher prevalence of elevated ALT compared with other ethnicities (71). Some individuals may have asymptomatic higher levels of AST probably related to a defect in enzyme clearance (72). Smoking appears not to cause any change with clinical significance in aminotransferase activity in serum independent of drinking (73,74). Coffee and caffeine consumption appear to reduce ALT activity in serum (75). AST and ALT activity in serum is altered by a great number of pharmacological and herbal agents (63).
De Ritis ratio: basics and interpretation
De Ritis ratio is a complex parameter that depends on AST and ALT activity in serum and physiological and pathological factors that determine their level. Since aminotransferase levels in serum are genetically-determined, De Ritis ratio has a heritability component. Two opposing factors control aminotransferase activities in serum: the rate (and kinetics) of release of aminotransferases from the liver and other organs and the clearance of enzymes from the circulation mostly by sinusoid liver cells (76). Although the activity of AST is higher than activity of ALT in almost all organs (from 2.5 times in the liver to 50 times in the brain relative to activity in serum; Figure 1), the differences in clearance rates between aminotransferases with a plasma half-life of AST approximately twice shorter than plasma half-life of ALT (50) lead to balanced levels of aminotransferases in serum and a De Ritis ratio of slightly less than 1 in healthy subjects (54). Elevation of De Ritis ratio may result from: (I) elevated AST activity (due to diseases leading to a greater release of AST than ALT); (II) reduced ALT activity alone or in combination with elevated AST (vitamin B6 deficiency; malnutrition, chronic renal failure, chronic alcoholism, liver aging); (III) a disproportionate elevation of AST activity when the activities of both enzymes are elevated (chronic alcohol consumption); (IV) a disproportional reduction of ALT activity when the activities of both enzymes are reduced (liver aging, uremia). The most common cause of a lower De Ritis ratio is elevated ALT activity in serum related to increased expression and/or release of the enzyme. Mild noxious stimuli may cause a mild hepatic cellular damage and release of cytoplasmic fraction (mostly ALT) which may decrease the De Ritis ratio whereas strong noxious stimuli may cause cellular destruction and release of cytoplasmic and mitochondrial fraction of aminotransferases (mostly AST) (43). In the latter scenario, an elevation of Re Ritis ratio is expected. De Ritis ratio may change in the course of disease. For instance, in early stages of NAFLD, the De Ritis ratio may be lower due to increased ALT activity (77) but at the fibrotic-cirrhotic stage, the De Ritis ratio tend to increase (78,79) due to hepatic cell destruction and AST release from mitochondria. In general, ALT is more specific (i.e., a negative test has a high probability to be truly negative) and AST is more sensitive (i.e., a positive test has a high probability to be truly positive) for mild liver damage. Muscular diseases and diseases with multi-organ failure are expected to lead to a higher De Ritis ratio due to AST release from large muscular mass or multiple sources. Diseases associated with lower or elevated De Ritis ratio are shown in Figure 1. The association of De Ritis ratio with CVD risk factors is discussed later in the review.
De Ritis ratio is easy to calculate from readily available components (aminotransferase measurements), yet it is a highly complex biochemical parameter and a non-standardized laboratory metric. Interpretation of the De Ritis ratio should be made in the specific clinical setting. Notably, there is no consensus with respect to the reference range of the De Ritis ratio and values from <1 (80) up to 1.3 in men (1.7 in women) (2) were proposed as reference range. In European adolescents supposed to have an ideal cardiovascular health, De Ritis ratio was 1.038±0.03 (obtained from mean ALT and AST values of 21.4±0.4 and 21.7±0.3 U/L, respectively) (81). The wide reference range of AST and ALT activities in serum makes it difficult to derive the reference range for the De Ritis ratio. Considering the reference range of AST (typically 10 to 40 U/L) and ALT (typically 10 to 50 U/L), theoretically a De Ritis ratio as low as 0.2 and as high as 4.0 may result from aminotransferase levels within the reference range. Although extreme low-high or high-low combinations of aminotransferases in serum are unlikely (42), the reference range of AST and ALT cannot exclude an abnormal De Ritis ratio. Abnormal aminotransferase levels and a De Ritis ratio >1 in patients with chronic viral hepatitis appear to predict poor outcomes and progression to cirrhosis (82,83). Studies using the receiver operating characteristic (ROC) curve analysis identified De Ritis ratio cu-offs >1.25 in patients with acute myocardial infarction (17), >1.40 in patients with stable coronary artery disease (CAD) (15) and >1.49 in patients with COVID-19 (18), as the best cut-offs regarding the prediction of mortality with the best tradeoff between sensitivity and specificity. Aminotransferase activity within the reference range does not necessarily reflect a healthy state. A true healthy ALT level ranging from 29 to 33 U/L for men and 19 to 25 U/L for women has recently been proposed (64) but it is too restrictive and needs confirmation. Recently, Valenti et al. (84) suggested ALT upper reference limits of 42/30 U/L in males/females, which were approximately 30% lower than International Federation of Clinical Chemistry (IFCC)-endorsed values. Unfortunately, such proposals have not been made for AST, which could have allowed a reappraisal of reference range of the De Ritis ratio. However, a recent study showed that AST activity between 15 and 24 U/L was associated with best survival in adults (85).
In the absence of a consensus with respect to reference values of the De Ritis ratio, 3 suggestions may be made regarding the interpretation of the De Ritis ratio. First, De Ritis ratio should be interpreted in conjunction with aminotransferase levels. A De Ritis ratio >1 in the presence of abnormal aminotransferase levels may indicate advanced liver damage (from various etiologies), prognosticate poor outcomes and it requires medical attention. Values >2.0 are highly indicative of alcohol-related liver disease (53,86). De Ritis ratio values >1 in patients with NAFLD or chronic viral hepatitis may indicate progression of the disease(s) to fibrosis or cirrhosis. A De Ritis ratio of ≤0.4 after severe hepato-toxicity from paracetamol poisoning appears to be highly predictive of recovery (87) potentially due to limited increase of AST release and consequently less severe hepatic damage. Even in patients with hepatitis A, a De Ritis ratio >1 was associated with higher risk of mortality with a dose-effect relationship (88). Second, a De Ritis ratio >1 even with AST and ALT activity within the reference range may indicate increased cardiometabolic risk and signify a poor prognosis (89). Whether De Ritis ratio values with aminotransferase levels within the healthy range provide prognostic risk is unclear and remains to be investigated. Third, if repeat aminotransferase measurements (with months to years in between) are available, changes over time in the De Ritis ratio may correlate with increasing metabolic risk (90) and thus they may need medical attention. One weakness of the De Ritis ratio is that in case of proportional elevations of AST and ALT, the De Ritis ratio may change little, and thus it cannot unmask the alterations (or the risk) associated with abnormal levels of aminotransferases. Moreover, there is a significant overlap between De Ritis ratio values in different diseases.
De Ritis ratio and CVD: epidemiological and clinical evidence
Evidence available strongly suggests a link between abnormal liver enzymes and cardiometabolic risk, CVD and mortality (22,23). The association between De Ritis ratio and CVD has been investigated to a lesser extent than the association of aminotransferases with CVD. Nevertheless, evidence available suggests an association between De Ritis ratio and CVD or CVD related mortality.
Cohort or population-based studies have investigated the link between De Ritis ratio and CVD or mortality (Table 1). Weng et al. (91) analyzed a prospective cohort of 29,316 primary care patients (in United Kingdom), 25–84 years of age with no history of CVD. Over a follow-up of 120,462 person-years, 782 patients (461 men and 321 women) experienced their first CVD event. The 10-year adjusted risk for the first CVD event was calculated based on 2 risk prediction tools (Framingham and QRISK2), with and without De Ritis ratio. The De Ritis ratio was significantly associated with the risk for the first CVD event in men [Framingham risk prediction: adjusted hazard ratios (HRs) =1.37, 95% confidence interval (CI): 1.05 to 1.79; QRISK2 risk prediction: adjusted HR =1.40 (1.04–1.89)] but not in women [Framingham risk prediction: adjusted HR =1.06 (0.78–1.43); QRISK2 risk prediction: adjusted HR =0.97 (0.70–1.35)] with all HRs calculated per unit higher log De Ritis ratio. The inclusion of De Ritis ratio in the model with Framingham risk factors [C-statistic: 0.72 (0.71–0.74)] or QRISK2 risk factors [C-statistic: 0.73 (0.71–0.74)] did not improve discrimination for CVD beyond the risk prediction tools. The authors concluded that elevated De Ritis ratio is associated with the increased risk of developing CVD in men but not in women and, that the De Ritis ratio did not offer improvement in the risk prediction over Framingham or QRISK2 risk tools. Nakajima et al. (92) performed an 8-year retrospective cohort study of 5,958 subjects aged 67–104 years. The link between De Ritis ratio and mortality was investigated using artificial intelligence and conventional analysis. Overall, 1,413 subjects (23.7%) died during the study. ALT, AST and De Ritis ratio were associated with the risk of mortality with adjusted HR =0.98 (0.97–0.99), 1.02 (1.02–1.03), and 1.46 (1.32–1.62), respectively, calculated per unit increment of each parameter (P<0.0001, for all 3 associations). The authors concluded that De Ritis ratio was strongly associated with mortality in elderly. Zoppini et al. (5) investigated the association between De Ritis ratio and all-cause and CVD mortality in 2,529 outpatients with diabetes mellitus over a 6-year follow-up. There were 305 deaths (12.1%) of which, 145 (47.5%) were CVD deaths. After adjustment for multiple demographical and clinical variables, De Ritis ratio, but not AST or ALT remained independently associated with the risk of all cause [adjusted HR =1.83 (1.14–2.93)] and CVD [adjusted HR =2.60 (1.38–4.90)] mortality. Diabetic patients with De Ritis ratio <1 were younger, had higher body mass index, higher glycosylated hemoglobin A1c, shorter duration of diabetes, higher glomerular filtration rate and triglyceride level and lower high-density lipoprotein-cholesterol than patients with De Ritis ratio >1. The study suggested that De Ritis ratio is superior to its components (AST and ALT) as a correlate of increased risk of all-cause or CVD mortality in patients with diabetes mellitus. Yokoyama et al. (93) performed a longitudinal cohort study of 3 494 Japanese subjects who participated in a community-based health check-up over a 10-year follow-up. The De Ritis ratio increased with increasing brain natriuretic peptide (BNP) levels and after adjustment it remained significantly associated with a high BNP level [defined as BNP ≥100 pg/mL; adjusted odds ratio (OR) =1.31 (1.13–1.53), per SD increase]. Overall, there were 250 deaths and 79 of them were CVD deaths. After adjustment, a high De Ritis ratio (>90 percentile) remained independently associated with the risk of all-cause mortality [adjusted HR =1.43 (1.04–1.96)] and CVD mortality [adjusted HR =2.51 (1.49–4.24)]. De Ritis ratio had an area under the ROC curve of 0.65 for CVD mortality. Subjects with a high De Ritis ratio were older, had a higher prevalence of CVD, lower prevalence of diabetes mellitus, lower body mass index, higher BNP and heart type fatty acid binding protein (H-FABP) levels and lower diastolic blood pressure, fasting blood glucose, glycosylated hemoglobin A1c and glomerular filtration rate compared with subjects with low De Ritis ratio. The authors concluded that De Ritis ratio is associated with higher BNP levels and increased CVD-mortality in general population and, that measuring De Ritis ratio during routine health check-ups may be a simple and cost-effective marker for prediction of CVD mortality. Katzke et al. (94) investigated the link between liver enzymes and De Ritis ratio with the incidence and mortality from CVD and 4 most common cancers (breast, prostate, colorectal and lung cancers) using a case-cohort sample of EPIC-Heidelberg case cohort study over an average follow-up of 15.6 years. There were 1,070 incident CVD events (555 incident myocardial infarctions and 515 incident strokes) and 381 CVD deaths over the follow-up. De Ritis ratio was not associated with incident CVD events including myocardial infarction [adjusted HR =0.90 (0.66–1.22)], stroke [adjusted HR =1.11 (0.82–1.50)] or CVD mortality (adjusted HR =1.07 (0.75–1.52)]. De Ritis ratio was independently associated with all-cause mortality [adjusted HR =1.40 (1.15–1.70)] and cancer-related mortality [adjusted HR =1.44 (1.15–1.80]. The study did not support a relationship between elevated liver enzymes or De Ritis ratio and major cardiovascular events or CVD mortality.
Table 1
| Author (year; reference) | Type of study | Number of participants (age) | Follow-up | De Ritis ratio cutoff | Outcome/adjusted risk estimate | Interpretation |
|---|---|---|---|---|---|---|
| Weng et al. [2015] (91) | Cohort study of primary care patients | 29,316 (25–84 yr) | 120,462 person/yrs | Unit of log Re Ritis ratio | Incident CVD: Framingham (M): HR =1.37 (1.05–1.79); QRISK2 (M): HR =1.40 (1.04–1.89); Framingham (W): HR =1.06 (0.78–1.43); QRISK2 (W): HR =0.97 (0.70–1.35) | Association with the risk of CVD in men but not in women |
| Nakajima et al. [2022] (92) | Retrospective cohort (elderly subjects) | 5,985 (67–104 yr) | 8 yr | Unit of De Ritis ratio | Mortality: HR =1.46 (1.32–1.62) | Association with mortality |
| Zoppini et al. [2016] (5) | Cohort study | 2,529 diabetic patients (70 yr) |
6 yr | Unit of De Ritis ratio | All-cause mortality: HR =1.83 (1.14–2.93); CVD mortality: HR =2.60 (1.38–4.90) | Association with all-cause and CVD mortality |
| Yokoyama et al. [2016] (93) | Longitudinal cohort study | 3,494 (62±10 yr) | 10 yr | Per SD increase | All-cause mortality: HR =1.43 (1.04–1.96) CVD mortality: HR =2.51 (1.49–4.24) |
Association with all–cause and CVD mortality |
| Katzke et al. [2020] (94) | Case cohort study | 25,546 (35–70 yr) | 15.6 yr | Unit of De Ritis ratio | MI: HR =0.90 (0.66–1.22); stroke: HR =1.11 (0.82–1.50); CVD death: HR =1.07 (0.75–1.52); all-cause death: HR = 1.40 (1.15–1.70) | No association with CVD or CVD mortality. Association with all-cause mortality |
| Liu et al. [2021] (95) | Cohort study | 14,220 hypertensive patients (63.80±9.36 yr) | 1.7 yr | Unit of De Ritis ratio | All-cause mortality: HR =1.37 (1.15–1.63); CVD mortality: HR =1.32 (1.03–1.68) | Association with all-cause and CVD mortality |
| Ferrannini et al. [2022] (96) | Pooled analysis of 2 randomized trials | 10,142 diabetic patients at high CVD risk (63.3 yr) | 2.4 yr | Unit of log Re Ritis ratio | Heart failure: HR =2.01 (1.06–3.68); heart failure or CVD death: HR =1.99 (1.26–3.10); all-cause death: HR =1.87 (1.11–3.07) | Association heart failure, composite of heart failure or CVD death and all-cause deaths |
| Liu et al. [2021] (97) | Multicenter prospective study | 6,527 patients with prior CAD events (57.8±10.9 yr) | 54.67±18.8 months | SD increase | All-cause death: HR =1.09 (1.00–1.19); CVD death: HR =1.13 (1.00–1.27); nonfatal MI: HR =0.98 (0.74–1.29); stroke: HR =1.08 (0.94–1,25) | Association with all-cause and CVD mortality. No association with MI or stroke |
| Ke et al. [2022] (8) | Population-based (elderly subjects) | 6,415 (≥65 yr) | 89 months | Higher vs. normal De Ritis ratio (1.0) | All-cause mortality: HR =1.68 (1.47–1.91); CVD mortality: HR =1.67 (1.27–2.20) | Association with all-cause and CVD mortality |
| Alexander et al. [2018] (98) | Case-cohort study | 572 patients with ischemic stroke; 1,017 subjects free of stroke | 5.8 yr | De Ritis ratio >2 | Stroke (whites): HR =2.74 (1.37–5.48); stroke (African Americans): HR =1.29 (0.70–2.40) | Association with stroke in whites. No association with stroke in African Americans |
CAD, coronary artery disease; CVD, cardiovascular disease; HR, hazard ratio; M, men; MI, myocardial infarction; yr, years; W, women.
Several studies have investigated the link of De Ritis ratio with prognosis in patients with CVD or at increased cardiovascular risk. Liu et al. (95) assessed the association between elevated De Ritis ratio and all-cause and CVD mortality in patients with arterial hypertension. The study included a cohort of 14,220 Chinese patients with arterial hypertension. There were 198 deaths (CVD deaths, 55.5%) over an average follow-up of 1.7 years. De Ritis ratio was associated with the increased risk of all-cause death [adjusted HR =1.37 (1.15–1.63)] and CVD death [adjusted HR =1.32 (1.03–1.68)], calculated per unit higher De Ritis ratio. The association between De Ritis ratio and all-cause or CVD mortality showed a similar strength in men and women without a De Ritis ratio-by-sex interaction regarding prediction of all-cause (Pint=0.731) or CVD (Pint=0.873) mortality. A recent study by Ferrannini et al. (96) that included 10,142 patients with diabetes mellitus at high cardiovascular risk enrolled in the CANVAS program showed that De Ritis ratio paralleled gamma-glutamyl transferase with respect to the association with the risk of heart failure [adjusted HR =2.01 (1.06–3.68)], composite of heart failure or CVD death [adjusted HR =1.99 (1.26–3.10)] or all-cause deaths [adjusted HR =1.87 (1.11–3.07)] over a 2.4-year follow-up (with all HRs calculated per unit higher log De Ritis ratio). Liu et al. (97) assessed the predictive value of liver fibrosis scores (including De Ritis ratio) for recurrent CVD events in a multicenter prospective study of 6 527 consecutive patients with angiography-diagnosed CAD who had experienced a prior CVD event [acute coronary syndrome, stroke, percutaneous coronary intervention (PCI), or coronary artery bypass grafting]. There were 532 recurrent CVD events (8.2%) over a mean follow-up of 54.67±18.80 months. De Ritis ratio correlated with the increased risk of total events [adjusted HR =1.09 (1.00–1.19)], CVD death [adjusted HR =1.13 (1.00–1.27)] but not the risk of nonfatal myocardial infarction [adjusted HR =0.98 (0.74–1.29)] or stroke [adjusted HR =1.08 (0.94–1.25)]. However, De Ritis ratio was a weaker correlate of recurrent CVD events compared with established liver fibrosis scores. Ke et al. (8) investigated the link between serum aminotransferases (and De Ritis ratio) and all-cause or cause specific mortality in 6,415 elderly subjects (≥65 years, without hepatic viral infection at baseline) obtained from the 1999–2014 National Health and Nutrition Examination Survey (NHANES) over a median follow-up of 89 months (range, 1–201 months). All-cause and CVD mortality occurred in 2,167 (33.8%) and 515 (23.8% of total deaths) participants. A high De Ritis ratio correlated significantly with all-cause [adjusted HR =1.68 (1.47–1.91)] and CVD [adjusted HR =1.67 (1.27–2.20)] mortality. De Ritis ratio showed the best utility for prediction of all-cause mortality in men and women. Alexander et al. (98) assessed the association between fatty liver index, liver enzymes and De Ritis ratio and the risk of stroke in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) cohort study over 5.8 years using a case-cohort of 572 cases of incident ischemic stroke and a stroke-free cohort random sample of 1,017 participants. African Americans were more likely to have a De Ritis ratio >2. A De Ritis ratio >2 was significantly associated with the risk of stroke in whites [adjusted HR =2.74 (1.37–5.48)] but not in African Americans [adjusted HR =1.29 (0.70–2.40]. There was no De-Ritis ratio-by-sex interaction (Pint=0.3) but there was a De-Ritis ratio-by-race interaction (Pint=0.03) regarding prediction of stroke in fully adjusted model. The inclusion of Framingham stroke risk factors increased the risk estimate for whites [HR =3.64 (1.42–9.35)] but not for African Americans [HR =0.97 (0.47–1.99)].
Liu et al. (99) performed a cross-sectional study to assess whether De Ritis ratio was associated with the prevalence of peripheral arterial disease (PAD) in 10,900 patients with arterial hypertension from the Chinese Hypertension Registry Study. The prevalence of PAD was 3.2% (n=350). After adjustment for demographical and clinical variables, De Ritis ratio was independently and positively associated with the prevalence of PAD [adjusted OR =1.31 (1.13–1.59)]. Another cross-sectional study by Rief et al. (100) investigated the link between De Ritis ratio and critical limb ischemia in 1,782 patients with PAD. An optimal cutoff of De Ritis ratio of 1.67 (sensitivity 34.1%, specificity 81%) was selected using the ROC curve analysis. Critical limb ischemia was more frequent among patients with De Ritis ratio >1.67 compared with patients with De Ritis ratio <1.67 (41.9% vs. 23.8%; P<0.001). After adjustment for well-established vascular risk factors, De Ritis ratio >1.67 remained independently associated with increased odds of critical limb ischemia [adjusted OR =2.0 (1.7–2.3)]. Patients with De Ritis ratio >1.67 were older, less often men, had lower body mass index, had more prevalent atrial fibrillation, congestive heart failure, CAD, renal failure and higher C-reactive protein and fibrinogen levels than patients with De Ritis ratio <1.67.
A number of studies have investigated the prognostic value of De Ritis ratio in patients with CAD. Liu et al. (15) assessed the association of De Ritis ratio with all-cause mortality in a retrospective cohort study of 203 patients with stable CAD over an average follow-up of 749 (435–1,122) days. Patients with a De Ritis ratio ≥1.40 (defined by ROC curve analysis) had significantly higher mortality [16.20% vs. 4.65%; adjusted HR =2.93 (1.08–7.91)]. A multicenter prospective study by Liu et al. (101) assessed the prognostic value of noninvasive liver fibrosis tests (including the De Ritis ratio) in 4,003 patients who underwent elective PCI over a mean follow-up of 5.0±1.6 years. There were 315 (7.87%) major cardiovascular events during the follow-up. De Ritis ratio was independently associated with the increased risk of cardiovascular events (cardiovascular death, nonfatal myocardial infarction or ischemic stroke) with an adjusted HR of 1.15 (1.04–1.27) per SD increase. Our group (102) assessed the association between De Ritis ratio and adverse events after PCI in 5,020 patients with stable CAD over a 3-year follow-up. At 3 years, all-cause deaths occurred in 5%, 7.5% and 14.5% of patients with the De Ritis ratio in the 1st (<0.75), 2nd (0.75 to 1.08) and 3rd (>1.08) tertile, respectively [adjusted HR =1.09 (1.06–1.12), P<0.001, per unit higher De Ritis ratio]. CVD deaths occurred in 3.0%, 4.5% and 8.0% of the patients with the De Ritis ratio in the 1st, 2nd and 3rd tertile, respectively [adjusted HR =1.09 (1.05–1.13), P<0.001, per unit higher De Ritis ratio]. The inclusion of De Ritis ratio in the multivariable models of mortality increased the C-statistic from 0.815 (0.794–0.836) to 0.818 (0.797–0.838) for all-cause mortality (P=0.005) and from 0.848 (0.822–0.874) to 0.855 (0.830–0.880) for CVD mortality (P=0.012). In addition, elevated De Ritis ratio correlated with increased risk of bleeding at 30 days and myocardial infarction and stroke at 3 years. The study suggested that De Ritis ratio improves discrimination for all-cause and CVD mortality and provides prognostic information on top of standard CVD risk factors. Steininger et al. (16) investigated whether aminotransferases and De Ritis ratio were associated with prognosis in 1,355 patients with acute myocardial infarction over a median follow-up of 8.6 years. There were 554 deaths (40.9%) over the follow-up including 414 CVD deaths. In unadjusted analysis, AST [unadjusted HR =1.19 (1.09–1.32)] and De Ritis ratio [unadjusted HR =1.31 (1.18–1.44)], both calculated per respective SDs, were associated with CVD mortality. ALT was not associated with the risk of mortality. After adjustment, the association between AST and mortality was attenuated whereas De Ritis ratio remained an independent correlate of CVD mortality [adjusted HR =1.23 (1.07–1.42), calculated per SD increment]. Similar findings were found for all-cause mortality. De Ritis ratio correlated positively with peak troponin and creatine kinase myocardial band, N-terminal probrain natriuretic peptide and C-reactive protein and negatively with glomerular filtration rate. The combination of De Ritis ratio with N-terminal probrain natriuretic peptide improved discrimination for mortality (net reclassification index and integrated discrimination improvement). Our group (17) investigated the association of De Ritis ratio with prognosis in 3,000 patients with acute myocardial infarction over a 3-year follow-up. At 3 years, all-cause deaths occurred in 13.2%, 17.8% and 21.9% of patients with De Ritis ratio in the 1st (<1.11), 2nd (1.11 to 1.95) and 3rd (>1.95) tertile [adjusted HR =1.16 (1.02–1.31) calculated per unit higher log De Ritis ratio]; CVD deaths occurred in 8.2%, 12.0% and 15.4% of patients in the respective tertiles of the De Ritis ratio (adjusted HR =1.20 [1.04–1.40] calculated per unit higher log De Ritis ratio). The inclusion of De Ritis ratio in the models of mortality did not increase significantly the C-statistic for all-cause (P=0.419) or CVD mortality (P=0.621). One study reported a significantly higher De Ritis ratio in patients presenting with ST-segment elevation acute myocardial infarction than in patients presenting with non-ST-segment elevation myocardial infarction and a De Ritis ratio ≥2.0 was a strong correlate of total coronary artery occlusion (103). A strong correlation between De Ritis ratio and cardiac troponin I (R=0.7) has been reported in patients presenting with acute coronary syndromes (104).
Two studies have assessed the link between elevated De Ritis ratio and outcomes of patients with acute ischemic stroke. A retrospective study of 421 patients with acute ischemic stroke by Gao et al. (6) showed that elevated De Ritis ratio was associated with poor outcomes at 3 months. A ROC curve analysis defined cutoff of De Ritis ratio of 1.53 was independently and positively associated with a poor outcome at 3 months [adjusted OR =1.89 (1.11–3.22)]. Xu et al. (105) assessed the link between De Ritis ratio and clinical outcome at 3 months and 1 year in 10,877 patients with acute ischemic stroke or transient ischemic attack enrolled in the Third China National Stroke Registry (CNSR-III). Patients with De Ritis ratio in the 4th quartile had higher risk of all-cause mortality within 3 months [adjusted HR =2.08 (1.25–3.47)] and 1 year [adjusted HR =2.26 (1.55–3.27)], and modified Rankin Score 3–6 and 2–6 at 1 year [adjusted OR =1.29 (1.07–1.55) and 1.20 (1.02–1.42), respectively], compared with patients with the De Ritis ratio in the first quartile. A multicenter retrospective study by Feng et al. (106) assessed the link between De Ritis ratio and CVD mortality in 1 579 patients on peritoneal dialysis who were followed up over 4,659.6 patient-years. There were 316 deaths (193 CVD deaths) over the follow-up. The high De Ritis ratio group (>1.0, a cutoff defined using the ROC curve analysis) had significantly higher CVD and all-cause mortality (24.5% vs. 15.2% and 32.6% vs. 20.7%, respectively) at 5 years of follow-up. After adjustment, the association of De Ritis ratio with CVD mortality [adjusted HR =1.43 (1.08–2.41)] and all-cause mortality [adjusted HR =1.45 (1.13–2.37)] remained significant. Older age, female sex, statin use and lower bilirubin were independent correlates of higher De Ritis ratio. Nam et al. (107) investigated the relationship between preoperative aminotransferases and 90-day mortality in 6,264 patients who underwent cardiovascular surgery. At 90 days, 183 patients (2.9%) died. After adjustment, low (≤13 IU/L) ALT level [adjusted HR =1.58 (1.14–2.18)] and high (>1.62) De Ritis ratio [adjusted HR =1.59 (1.15–2.20)] were independently associated with mortality compared with respective middle values (13–30 U/L for ALT and 0.85–1.62 for the De Ritis ratio). High [adjusted HR =1.39 (0.96–2.01)] or low AST [adjusted HR =1.31 (0.91–1.89)] levels were not associated with mortality compared with middle values (17–30 U/L). The association of low ALT or high De Ritis ratio with 90-day mortality was more pronounced in patients older than 60 years (Pint <0.05 for both interactions).
Numerous recent studies have assessed the De Ritis ratio in morbid conditions associated with CVD or CVD related adverse events. De Ritis ratio has been shown to be associated with sarcopenia (108,109), frailty in older patients with heart failure (10), nutritional status and worse clinical outcomes in patients with acute heart failure (110), rhabdomyolysis (111), coronary lesions in Kawasaki disease (97,112), epicardial fat (113), contrast-induced acute kidney injury after elective PCI (114), acute kidney injury (115), hemolysis or devise thrombosis (116) and mortality (117) after implantation of left ventricular assist devices, acute kidney injury after cardiac surgery (118), functional severity of chronic heart failure with reduced left ventricular ejection fraction (119), unfavorable prognosis in patients in early stage of severe fever with thrombocytopenia syndrome (120), wider pulse pressure and increased odds of arterial hypertension (4), arterial stiffness (121), microvascular angina pectoris (122), coronary slow flow (123), mortality after cardiac arrest (9), in-hospital mortality in patients with pulmonary embolism (124), mortality in patients with pulmonary arterial hypertension (125) or thoracoabdominal trauma (126). The association of De Ritis ratio with these morbid conditions may show that an elevated De Ritis ratio is a correlate of severity of the condition and consequently of subsequent adverse events.
In aggregate, the current status of knowledge with respect to the evidence linking De Ritis ratio with the CVD or mortality may be summarized as follows: (I) The evidence on the association between De Ritis ratio and incident CVD is strongly suggestive but not conclusive mostly due to limited longitudinal population-based studies in this field. (II) Epidemiological and clinical studies strongly support an association between elevated De Ritis ratio and the risk of all-cause and CVD mortality. (III) Whether the association between De Ritis ratio and CVD or mortality differs according to age, gender and ethnicity or whether the De Ritis ratio provides prognostic information on top of traditional cardiovascular risk factors requires further investigation.
De Ritis ratio and CVD: underlying mechanisms
Although, high and low De Ritis ratio may be associated with cardiometabolic risk and CVD, the majority of studies have evidenced an association between elevated De Ritis ratio and these outcomes. The underlying mechanisms of the association of elevated De Ritis ratio with CVD or mortality are not entirely clear. However, based on the existing studies, a number of mechanisms may be offered.
Elevated De Ritis ratio due to elevated AST level
Chronic liver disease affecting approximately 1.5 billion people worldwide in 2017 (127) is a common cause of the elevated AST level and De Ritis ratio. In fact, liver is the most important determinant of AST activity in health and disease and an elevated AST indicates an advanced stage of liver disease. Mechanisms of AST release from the cells (including hepatocytes) are discussed earlier in this review (see: Biology of aminotransferases). CVD is a leading cause of morbidity and mortality in patients in the end-stage of chronic liver disease (128). CAD is frequent in patients with end-stage chronic liver disease with a prevalence of up to 36.8% at the time of liver transplant (129). Chronic liver diseases like NAFLD (130) and nonalcoholic steatohepatitis (131) have increased cardiovascular risk for atherosclerosis and CAD even at the pre-cirrhotic stage and up to 11.6% of PCI procedures are performed in patients with a formal diagnosis of cirrhosis (132). Chronic liver disease is associated with higher odds of major adverse cardiovascular and cerebrovascular events, mortality and major bleeding, contrast-induced acute renal injury, longer hospitalization and increased costs after PCI (122,133,134). Patients with known advanced chronic liver disease are less likely to undergo invasive treatment due to fears of increased risk of complications (135). It should be recognized, however, that most patients with chronic liver disease remain asymptomatic up to advanced stages of the disease and are discovered incidentally during medical visits for other reasons (136). NAFLD is most common liver disease with an estimated prevalence of 32.4% worldwide (137). In early stages of disease liver enzymes may be mildly elevated, particularly ALT, which leads to a De Ritis ratio of less than one (78,138). However, with the disease progression towards fibrosis, reversal of De Ritis ratio may occur due to increased mitochondrial damage leading to release of mitochondrial fraction of AST and decreased hepatic clearance (139), which portends a poor prognosis (140). Reasonably, an elevated AST level and De Ritis ratio in the setting of liver disease is expected to be associated with higher odds of CVD and a poor prognosis.
Chronic alcohol consumption is a major cause of elevated AST activity in serum and De Ritis ratio. Alcohol damages liver via multiple mechanisms and liver bears the greatest degree of tissue injury by heavy drinking because it is the primary site of ethanol metabolism (141). As already mentioned in this review, De Ritis ratio values ≥2 indicate higher odds of alcohol-related liver disease (53,86) and Nyblom et al. (53) correctly pointed out that an elevated De Ritis ratio is a marker of alcohol-related liver damage rather than of heavy drinking. Increased AST activity by chronic alcohol consumption is expainable considering destructive actions of alcohol on hepatocytes (141). In addition, alcohol itself stimulates the synthesis and release of mitochondrial AST, thereby increasing the De Ritis ratio (142). Serum activity of the mitochondria1 AST is strikingly higher in alcoholic subjects than healthy controls and the mean mitochondrial AST to total AST ratio is 4 times higher in alcoholic subjects (52). One liver biopsy study showed increased amounts of mitochondrial AST messenger RNA indicating a possible increase in the total production of mitochondrial AST in the liver induced by alcohol (143). Although, alcohol has complex effects on cardiovascular health, heavy and prolonged drinking increases the risk for developing CVD and is associated with a poor prognosis (144,145). The alcohol-related deficiency pyridoxal-5’-phosphate is discussed later in this review.
Elevation of AST activity in serum and De Ritis ratio occur in acute coronary syndromes due to AST release from ischemic or necrotic myocardium. Historically, measurement of AST activity in serum was the first biochemical test used to diagnose acute myocardial infarction (146). Since AST activity is highest in myocardium (Figure 1), the release of AST from ischemic/necrotic myocardium is large enough to increase AST activity in serum and consequently the De Ritis ratio with values typically >1.3. AST activity—an enzymatic marker of myocardial necrosis (147,148)—increases within the first 6 hours after AMI onset (104). In the setting of acute coronary syndromes or acute myocardial infarction, De Ritis ratio has been reported to correlate with peak troponin and creatine kinase myocardial band levels (16,104). In patients with acute myocardial infarction, De Ritis ratio has been shown to be a better prognostic marker than its components, AST and ALT (16,17). The strong correlation between elevated De Ritis ratio and established markers of myocardial necrosis may explain, the prognostic value of De Ritis ratio in patients with acute coronary syndromes. Skeletal muscle disease of various etiologies and hematological disorders are also common causes of elevated AST activity and De Ritis ratio.
Elevated De Ritis ratio is common in morbid conditions characterized by multiorgan involvement or failure. Multi-organ injury may lead to increased release of AST from multiple sources elevating AST activity in serum and De Ritis ratio (Figure 1). In conditions of prolonged circulatory insufficiency or increased metabolic or toxic stress in general, elevated De Ritis ratio indicates disease severity and end-organ damage that heralds a poor prognosis. A gradual increase in the frequency of cardiogenic shock from lower to upper tertiles of the De Ritis ratio was reported in patients with acute myocardial infarction (17) and explained by prolonged circulatory insufficiency leading to end-organ damage (16). Elevated De Ritis ratio was reported in patients with out-of-hospital cardiac arrest (9), a morbid condition characterized by prolonged and generalized hypoperfusion and widespread end-organ damage. Ischemic hepatitis developing in the setting of prolonged circulatory insufficiency may be an important contributing factor to elevated De Ritis ratio and poor prognosis (149-151). Low cardiac output with reduction of hepatic blood flow may lead to ischemic hepatitis, even without shock (152,153). Ischemic hepatitis is characterized by a rapid rise-and-fall pattern of AST activity in serum (154). A high De Ritis ratio correlates with poor prognosis in other morbid conditions characterized with multiorgan involvement (or failure) like major burns (13), sepsis and septic shock (14,155), and polymyositis/dermatomyositis-associated interstitial lung disease (156).
Finally, the possibility that an elevated AST activity and De Ritis ratio may reflect a metabolic trait that predisposes to future metabolic derangements even in absence of overt clinical disease should be discussed. As suggested by Sookoian et al. (21), elevation of aminotransferase activity in metabolic diseases like NAFLD may indicate basic metabolic abnormalities at the amino acid and Krebs cycle levels. The most important cellular stimuli that control (induce) AST expression are those associated with increased gluconeogenesis (32,33). Notably, the localization of both AST and gluconeogenic enzymes in the same (periportal) zone of hepatic acinus further strengthened the evidence that AST and gluconeogenesis are closely linked. Cytoplasmic AST is also involved in glyceroneogenesis (38). Based on these lines of evidence, we hypothesize that an elevated AST and De Ritis ratio even in absence of clinical disease may indicate basic metabolic abnormalities or a metabolic trait that may serve as a cradle of future metabolic diseases.
Elevated De Ritis ratio due to reduced ALT level
Reduced ALT activity in serum may lead to elevated De Ritis ratio. In this scenario, prognostic information mediated by elevated De Ritis ratio may be different from the information conferred by elevated De Ritis ratio due to elevated AST. A reduction in ALT activity occurs with advancing age (157) and appears to be due to age-related liver alterations characterized by reduced liver size and blood flow and histological alterations presumably due to prolonged oxidative stress (158,159). An inverted U-shaped relationship between age and ALT activity in serum with lower ALT activity at younger and older ages and a peak activity at 40–55 years has been reported (160). Liver diseases with extensive fibrosis involving large parts of liver parenchyma may lead to low ALT due to reduced production and release of the enzyme, commonly associated with indicators of reduced hepatic function such as low albumin and cholesterol. Frailty (157) and loss of independence (161) are associated with low ALT activity in serum, which may be explained by mechanisms similar to those explaining ALT lowering with aging as well as by diseases commonly associated with these morbid conditions, particularly in elderly. Lower ALT activity in serum is often found in patients with sarcopenia (162), which correlates with reduced survival. Lower ALT in individuals ≥65 years old who are free of chronic liver disease, cancer or excessive alcohol consumption is an indicator of frailty, disability, and sarcopenia and an independent correlate of reduced survival (163). Worse nutritional state and pyridoxal-5’-phosphate deficiency may contribute to low ALT activity levels and poor survival (158). Lower ALT activity may reflect a poor nutritional state (158). Pyridoxal-5’-phosphate deficiency, either isolated or in the context of poor nutritional state may lead to lower ALT activity and poor outcomes (163). If low ALT in serum reflects a low ALT activity in cells, then reduced rates of transamination may occur and lead to lower rates of gluconeogenesis and reduced oxidative capacity of the cells. Reduced testosterone level may lead to low ALT activity, either through androgen involvement in ALT expression (34) or in the setting of frailty syndrome (164) in men. Reduced testosterone levels are common in elderly and correlate with markers of atherosclerosis in patients with diabetes (165) and reduced survival in patients with CAD (166). Thus, elevated De Ritis ratio occurring in the setting of lower ALT activity reflects cardiometabolic risk conferred by conditions associated with low ALT, which contribute to the understanding of the link of higher De Ritis ratio with poor prognosis and increased risk of CVD mortality.
Alcohol consumption may lower pyridoxal-5’-phosphate levels and alter the aminotransferase activity. Acetaldehyde—an intermediary product of alcohol metabolism (a product of alcohol dehydrogenase)—accelerates pyridoxal-5’-phosphate decay by displacing it from binding proteins, which protects the coenzyme against hydrolysis (167). Free pyridoxal-5’-phosphate is dephosphorylated by alkaline phosphatase (or pyridoxal phosphatase) producing pyridoxal which is degraded further by aldehyde oxidase to pyridoxic acid—the main degradation product of vitamin B6 metabolism (168). It has been suggested that deficiency of pyridoxal 5’-phosphate decreases ALT synthesis to a greater extent than AST synthesis (142). A recent genome-wide association study showed that elevated De Ritis ratio in subjects with moderate-to-high drinking behavior and the rs671 GA genotype was due to decreased levels of ALT not accompanied with significant change in the AST levels. Although there was an interaction effect in both men and women, the effect was larger in men (169). The rs671 is a non-synonymous G-to-A transition (leading to Glu504-to-Lys substitution) in an aldehyde dehydrogenase 2 protein-coding region of the aldehyde dehydrogenase 2 gene that is associated with almost a total loss of enzyme activity (158). If pyridoxal-5’-phosphate deficiency at cellular level leads to reduced rates of transamination reactions, then signaling pathways to increase the expression and production of aminotransferases may be activated. In the setting of coenzyme deficiency, this may lead to increased levels of hollow enzymes (afunctional apoenzyme without prosthetic group, i.e., pyridoxal-5’-phosphate) in sera of alcoholic subjects which may have implications for aminotransferase assays with pyridoxal-5’-phosphate activation. However, this hypothesis needs testing. One autopsy study of 20 patients showed that pyridoxal-5’-phosphate activation increased the activity of AST to a greater extent than activity of ALT (compared with values without pyridoxal-5’-phosphate activation) across various human tissues including liver, heart, skeletal muscle, lung, spleen, kidney, duodenum and brain (170).
De Ritis ratio and cardiovascular risk factors
The association between circulating aminotransferases and cardiometabolic risk factors is complex and has been recently reviewed (22,23). Population-based studies have shown that elevated aminotransferase levels are associated with several cardiovascular risk factors including arterial hypertension, diabetes mellitus, metabolic syndrome, abdominal obesity, triglyceride level, impaired fasting glucose and insulin resistance (171-175). However, De Ritis ratio may behave differently from aminotransferases in terms of association with cardiometabolic risk factors.
Population-based studies (5,95,97) and studies in patients with CAD (16,17) have shown that an elevated De Ritis ratio is more likely to be associated with old age and female sex. The increase in the De Ritis ratio with advancing age may be due to progressive reduction of ALT activity with aging. Elevated De Ritis ratio correlates strongly with impaired renal function as assessed by estimated glomerular filtration rate (5,95,97). The lowering of aminotransferase activity in serum is proportional to the reduction of the glomerular filtration rate in patients with chronic kidney disease (176) and is explainable by vitamin B6 deficiency, hemodilution, uremic toxins and hyperhomocysteinemia that characterize chronic kidney disease (177-180). Although a lower De Ritis ratio is plausible in chronic kidney disease, consistent reports of an inverse association between De Ritis ratio and glomerular filtration rate suggest a greater likelihood of an elevated Re Ritis ratio associated with chronic kidney disease. In this scenario, elevation of De Ritis ratio may be explainable by reduced ALT activity or increased AST release due to increased stress (toxic, oxidative, inflammatory) on multiple organs and systemic alterations occurring in the setting of chronic kidney disease. Chronic kidney disease is well-known risk factor for CVD and CVD mortality. An elevated De Ritis ratio due to chronic kidney disease may reflect metabolic disarrangements and increased CVD risk. An inverse association between De Ritis ratio and obesity assessed by body mass index was consistently reported (5,16,17,95,97). This pattern of relationship is explainable considering increased De Ritis ratio in elderly, sarcopenic and frail subjects and subjects with malnutrition and vitamin B6 deficiency, all of them known to be associated with lower body mass index. Increased ALT activity in conditions associated with obesity (diabetes, metabolic syndrome, hepatic steatosis and NAFLD) appears to further contribute to the inverse association between obesity and the De Ritis ratio. An elevated De Ritis ratio appears to be associated with systemic inflammation assessed by C-reactive protein in patients with PAD (100), acute myocardial infarction (16) and peritoneal dialysis (106). Our group reported a U-shaped relationship between C-reactive protein and the De Ritis ratio, with higher values of C-reactive protein in lower and upper tertiles of De Ritis ratio compared with the middle tertile. This relationship may be due to liver disease and obesity being more frequent among patients with the lower De Ritis ratio and older age, reduced body mass index and reduced renal function being more frequent among patients with the higher De Ritis ratio (17).
Several studies have shown an inverse relationship between De Ritis ratio and the incidence of metabolic syndrome and diabetes mellitus. Of 633 subjects from the Insulin Resistance Atherosclerosis Study who were free of metabolic syndrome, 127 subjects developed metabolic syndrome after 5.2 years of follow-up. Subjects in the higher quartile of the De Ritis ratio had a lower risk of developing metabolic syndrome [adjusted HR =0.48 (0.25–0.95)]. The association was not modified by ethnicity or sex and remained significant following exclusion of former and heavy drinkers (181). A population-based cohort study that included 2,276 adults (903 men and 1,373 women) aged 40–70 years without metabolic syndrome showed that 395 subjects (17.4%) developed metabolic syndrome over an average 2.6-year follow-up. After adjustment, subjects with De Ritis ratio in the 4th quartile had significantly lower risk of developing metabolic syndrome compared with subjects in the 1st quartile [adjusted HR =0.598 (0.422–0.853)]. In this study, De Ritis ratio had incremental predictive value for incident metabolic syndrome (182). A recent study in Chinese adolescents showed that De Ritis ratio was inversely associated with waist circumference, waist-to-hip ratio, body mass index, diastolic blood pressure, triglyceride and low-density lipoprotein level, uric acid, fasting insulin and insulin resistance and directly with high-density lipoprotein level. In this study, the risk of metabolic syndrome was approximately 6 times higher among adolescents with lowest versus highest De Ritis ratio over 5 years of follow-up (183). A large population-based study (n=70,688 subjects) showed a significant association between low De Ritis ratio and incident diabetes over 10 years of follow-up. The risk of diabetes was higher in subjects with De Ritis ratio ≤0.875, ALT ≥23 U/L and body mass index <25 kg/m2 compared with subjects with De Ritis ratio >0.875, ALT <23 U/L and body mass index ≥25 kg/m2 (184). A retrospective cohort study of 15,464 subjects in Japan, showed an inverse relationship between De Ritis ratio and the risk of diabetes mellitus up to a value of 0.93 [adjusted HR =0.14 (0.02–0.90)] whereas for De Ritis ratio values >0.93 the risk for developing diabetes was not significant [adjusted HR =0.67 (0.17–2.65)] over a median follow-up of 5.38 years (185). Similarly, a retrospective cohort study reported an inverse relationship between De Ritis ratio and the risk of diabetes mellitus for De Ritis ratio values up to 0.882 (186). In patients with diabetes mellitus, elevated De Ritis ratio was independently associated with the risk of progression of diabetic nephropathy (186). One study in patients with arterial hypertension has reported an inverse association between De Ritis ratio and low-density lipoprotein-cholesterol, triglycerides, albumin and uric acid and a positive correlation with high-density lipoprotein-cholesterol and homocysteine (95). The association between De Ritis ratio and low-density lipoprotein-cholesterol remains controversial. De Ritis ratio appears to be inversely associated with gamma-glutamyl transferase (95).
In aggregate, evidence available suggests that the association between elevated De Ritis ratio and cardiovascular risk factors is diverse and not particularly strong. Elevated De Ritis ratio correlates positively with age, systemic inflammation and impaired renal function and inversely with obesity, metabolic syndrome and diabetes mellitus. The extent and direction of the association between De Ritis ratio and cardiovascular risk factors cannot explain the consistently reported strong association between elevated De Ritis ratio and CVD or CVD mortality. In an attempt to explain the link between elevated De Ritis ratio and CVD and CVD mortality, we hypothesize that De Ritis ratio absorbs and mediates cardiovascular risk that is not provided by standard cardiovascular risk factors. In this regard, De Ritis ratio may be considered as an emerging nonstandard cardiometabolic risk marker.
Low De Ritis ratio
The majority of studies summarized in this review showed an association between elevated De Ritis ratio and CVD or CVD mortality. However, a lower De Ritis ratio correlates with liver disease, obesity and dysmetabolic states in the setting of inverse association with these conditions (5,16,17,95,97,181-186) and may contain cardiometabolic risk. A low De Ritis ratio may result from low AST levels associated with vitamin B6 deficiency (187). Low concentration of vitamin B6 in plasma is more likely to be found in association with elevated alkaline phosphatase in elderly and patients with advanced renal, hepatic or inflammatory diseases (187). Lower AST levels are found in chronic kidney disease patients and AST levels decrease further with progression of the disease (180).
Elevated ALT levels and consequently a lower De Ritis ratio may occur in numerous morbid conditions including highly prevalent liver disease, particularly NAFLD considered to be a strong correlate or even an equivalent of metabolic syndrome. Elevated ALT level correlates positively with liver fat estimated by magnetic resonance spectroscopy (188,189). ALT correlates with insulin resistance in liver, insulin secretion, and glucagon level in healthy subjects of both sexes (190). Elevated ALT correlates positively with atherogenic lipids (apolipoprotein B, triglycerides and small dense low-density lipoprotein particles), plasma glucose, C-reactive protein, abdominal obesity and arterial hypertension and negatively with apolipoprotein A1 and high-density lipoprotein-cholesterol (191-195). Elevated ALT is associated with plasminogen activator inhibitor-1 antigen, coagulation factor XIII B subunit, and factor XII (suggesting a higher thrombotic risk) (194), Framingham risk score (196,197), endothelial dysfunction (198), coronary calcification (199), coronary atherosclerosis (200,201) and lower adiponectin levels (202). Morbid conditions associated with elevated ALT activity and association with cardiovascular risk have been recently reviewed (22). With this evidence in background, it remains puzzling why elevated but not lower De Ritis ratio correlates with CVD and CVD mortality in most studies. Although the underlying mechanisms remain unclear, a concomitant increase in the AST level in these conditions may counterbalance the impact of elevated ALT level on De Ritis ratio offsetting the association between conditions associated with elevated ALT level and CVD or CVD mortality. Likewise, concomitant elevation of AST levels (if occurs) may reflect a more severe condition (or stage of the disease) than elevated ALT level alone, and consequently has a higher impact on prognosis.
Conclusions
De Ritis ratio is an emerging cardiometabolic risk marker. Although easy to calculate from readily available components, the De Ritis ratio is a highly complex biochemical parameter that needs to be standardized. De Ritis ratio absorbs a large amount of cardio-metabolic information that is not mediated by individual aminotransferases. Abnormal values of De Ritis ratio may indicate increased cardiometabolic risk associated with occult or overt hepatic or extrahepatic diseases and in absence of overt clinical disease, they (particularly elevated values) may represent a metabolic trait that foresees future metabolic disease(s). Although low and high De Ritis ratio may be associated with cardiometabolic risk, the majority of studies support an association between elevated De Ritis ratio and prognosis. Recent evidence suggests a consistent association of De Ritis ratio with prognosis across various diseases, including diabetes mellitus, cancer, diseases characterized by multiorgan failure, CVD, stroke and COVID-19. Evidence linking De Ritis ratio with the risk of incident CVD is suggestive but not definitive due to limited epidemiological evidence from high-quality longitudinal studies of sufficient duration. Epidemiological and clinical studies strongly support an association of elevated De Ritis ratio with the risk of total and CVD mortality. The majority of studies supporting an association between De Ritis ratio and CVD or CVD mortality came from Asian populations. The association between De Ritis ratio and traditional cardiovascular risk factors is diverse and not strong enough to explain the association of De Ritis ratio with CVD or CVD mortality. It is likely that De Ritis ratio absorbs and mediates cardiometabolic risk that is poorly incorporated in and mediated by traditional cardiovascular risk factors—an attribute that may explain its association with CVD and CVD mortality. In this regard, De Ritis ratio may be considered as a nonstandard cardiometabolic marker. Whether the strength of association between De Ritis ratio and CVD or mortality differs according to ethnicity, age and sex requires further investigation. The De Ritis ratio requires standardization by authoritative bodies with respect to reference range and interpretation. Future epidemiological, clinical and laboratory studies are required to further clarify the association between De Ritis ratio and CVD and related prognosis.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-22-68/coif). GN serves as an unpaid editorial board member of Journal of Laboratory and Precision Medicine from July 2020 to July 2024. The author has no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- De Ritis F, Coltorti M, Giusti G. An enzymic test for the diagnosis of viral hepatitis; the transaminase serum activities. Clin Chim Acta 1957;2:70-4. [Crossref] [PubMed]
- Botros M, Sikaris KA. The de ritis ratio: the test of time. Clin Biochem Rev 2013;34:117-30. [PubMed]
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 2002;346:1221-31. [Crossref] [PubMed]
- Long MT, Pedley A, Massaro JM, et al. The Association between Non-Invasive Hepatic Fibrosis Markers and Cardiometabolic Risk Factors in the Framingham Heart Study. PLoS One 2016;11:e0157517. [Crossref] [PubMed]
- Zoppini G, Cacciatori V, Negri C, et al. The aspartate aminotransferase-to-alanine aminotransferase ratio predicts all-cause and cardiovascular mortality in patients with type 2 diabetes. Medicine (Baltimore) 2016;95:e4821. [Crossref] [PubMed]
- Gao F, Chen C, Lu J, et al. De Ritis ratio (AST/ALT) as an independent predictor of poor outcome in patients with acute ischemic stroke. Neuropsychiatr Dis Treat 2017;13:1551-7. [Crossref] [PubMed]
- He HM, He C, Zhang SC, et al. Predictive value of aspartate aminotransferase-to-alanine aminotransferase ratio for contrast-associated acute kidney injury in patients undergoing elective percutaneous coronary intervention. J Cardiol 2022;79:618-25. [Crossref] [PubMed]
- Ke P, Zhong L, Peng W, et al. Association of the serum transaminase with mortality among the US elderly population. J Gastroenterol Hepatol 2022;37:946-53. [Crossref] [PubMed]
- Lu Z, Ma G, Chen L. De-Ritis Ratio Is Associated with Mortality after Cardiac Arrest. Dis Markers 2020;2020:8826318. [Crossref] [PubMed]
- Maeda D, Kagiyama N, Jujo K, et al. Aspartate aminotransferase to alanine aminotransferase ratio is associated with frailty and mortality in older patients with heart failure. Sci Rep 2021;11:11957. [Crossref] [PubMed]
- Su S, Liu L, Li C, et al. Prognostic Role of Pretreatment De Ritis Ratio (Aspartate Transaminase/Alanine Transaminase Ratio) in Urological Cancers: A Systematic Review and Meta-Analysis. Front Oncol 2020;10:1650. [Crossref] [PubMed]
- Yu J, Kim HY, Kong YG, et al. De Ritis ratio as a predictor of 1-year mortality after burn surgery. Burns 2021;47:1865-72. [Crossref] [PubMed]
- Wang B, Hu L, Chen Y, et al. Aspartate transaminase/alanine transaminase (De Ritis ratio) predicts survival in major burn patients. Burns 2022;48:872-9. [Crossref] [PubMed]
- Zhao PY, Yao RQ, Ren C, et al. De Ritis Ratio as a Significant Prognostic Factor in Patients with Sepsis: A Retrospective Analysis. J Surg Res 2021;264:375-85. [Crossref] [PubMed]
- Liu X, Liu P. Elevated AST/ALT ratio is associated with all-cause mortality in patients with stable coronary artery disease: a secondary analysis based on a retrospective cohort study. Sci Rep 2022;12:9231. [Crossref] [PubMed]
- Steininger M, Winter MP, Reiberger T, et al. De-Ritis Ratio Improves Long-Term Risk Prediction after Acute Myocardial Infarction. J Clin Med 2018; [Crossref] [PubMed]
- Ndrepepa G, Holdenrieder S, Kastrati A. Prognostic value of De Ritis ratio in patients with acute myocardial infarction. Clin Chim Acta 2022; Epub ahead of print. [Crossref] [PubMed]
- Zinellu A, Arru F, De Vito A, et al. The De Ritis ratio as prognostic biomarker of in-hospital mortality in COVID-19 patients. Eur J Clin Invest 2021;51:e13427. [Crossref] [PubMed]
- Braunstein AE, Kritzmann MG. Decomposition and synthesis of amino acids by conversion of amines; studies on muscle tissue. Enzymologia 1937;2:129-46.
- Sookoian S, Pirola CJ. Liver enzymes, metabolomics and genome-wide association studies: from systems biology to the personalized medicine. World J Gastroenterol 2015;21:711-25. [Crossref] [PubMed]
- Sookoian S, Castaño GO, Scian R, et al. Serum aminotransferases in nonalcoholic fatty liver disease are a signature of liver metabolic perturbations at the amino acid and Krebs cycle level. Am J Clin Nutr 2016;103:422-34. [Crossref] [PubMed]
- Ndrepepa G, Kastrati A. Alanine aminotransferase—a marker of cardiovascular risk at high and low activity levels. J Lab Precis Med 2019;4:29. [Crossref]
- Ndrepepa G. Aspartate aminotransferase and cardiovascular disease—a narrative review. J Lab Precis Med 2021;6:6. [Crossref]
- Panteghini M. Aspartate-Aminotransferase Isoenzymes. Clin Biochem 1990;23:311-9. [Crossref] [PubMed]
- Hayashi H, Wada H, Yoshimura T, et al. Recent topics in pyridoxal 5'-phosphate enzyme studies. Annu Rev Biochem 1990;59:87-110. [Crossref] [PubMed]
- Pol S, Bousquet-Lemercier B, Pavé-Preux M, et al. Chromosomal localization of human aspartate aminotransferase genes by in situ hybridization. Hum Genet 1989;83:159-64. [Crossref] [PubMed]
- Glinghammar B, Rafter I, Lindström AK, et al. Detection of the mitochondrial and catalytically active alanine aminotransferase in human tissues and plasma. Int J Mol Med 2009;23:621-31. [Crossref] [PubMed]
- Sohocki MM, Sullivan LS, Harrison WR, et al. Human glutamate pyruvate transaminase (GPT): localization to 8q24.3, cDNA and genomic sequences, and polymorphic sites. Genomics 1997;40:247-52. [Crossref] [PubMed]
- Yang RZ, Blaileanu G, Hansen BC, et al. cDNA cloning, genomic structure, chromosomal mapping, and functional expression of a novel human alanine aminotransferase. Genomics 2002;79:445-50. [Crossref] [PubMed]
- Yang RZ, Park S, Reagan WJ, et al. Alanine aminotransferase isoenzymes: molecular cloning and quantitative analysis of tissue expression in rats and serum elevation in liver toxicity. Hepatology 2009;49:598-607. [Crossref] [PubMed]
- Lindblom P, Rafter I, Copley C, et al. Isoforms of alanine aminotransferases in human tissues and serum--differential tissue expression using novel antibodies. Arch Biochem Biophys 2007;466:66-77. [Crossref] [PubMed]
- ROSEN F. ROBERTS NR, NICHOL CA. Glucocorticosteroids and transaminase activity. I. Increased activity of glutamicpyruvic transaminase in four conditions associated with gluconeogenesis. J Biol Chem 1959;234:476-80. [PubMed]
- Begum NA, Datta AG. Effect of adrenergic agonists and antagonists on alanine amino transferase, fructose-1:6-bisphosphatase and glucose production in hepatocytes. Mol Cell Biochem 1992;113:93-103. [Crossref] [PubMed]
- Coss CC, Bauler M, Narayanan R, et al. Alanine aminotransferase regulation by androgens in non-hepatic tissues. Pharm Res 2012;29:1046-56. [Crossref] [PubMed]
- Aggerbeck M, Garlatti M, Feilleux-Duché S, et al. Regulation of the cytosolic aspartate aminotransferase housekeeping gene promoter by glucocorticoids, cAMP, and insulin. Biochemistry 1993;32:9065-72. [Crossref] [PubMed]
- Thulin P, Rafter I, Stockling K, et al. PPARalpha regulates the hepatotoxic biomarker alanine aminotransferase (ALT1) gene expression in human hepatocytes. Toxicol Appl Pharmacol 2008;231:1-9. [Crossref] [PubMed]
- Edgar AD, Tomkiewicz C, Costet P, et al. Fenofibrate modifies transaminase gene expression via a peroxisome proliferator activated receptor alpha-dependent pathway. Toxicol Lett 1998;98:13-23. [Crossref] [PubMed]
- Tordjman J, Leroyer S, Chauvet G, et al. Cytosolic aspartate aminotransferase, a new partner in adipocyte glyceroneogenesis and an atypical target of thiazolidinedione. J Biol Chem 2007;282:23591-602. [Crossref] [PubMed]
- Thulin P, Bamberg K, Buler M, et al. The peroxisome proliferator-activated receptor α agonist, AZD4619, induces alanine aminotransferase-1 gene and protein expression in human, but not in rat hepatocytes: Correlation with serum ALT levels. Int J Mol Med 2016;38:961-8. [Crossref] [PubMed]
- Kalousdian S, Fabsitz R, Havlik R, et al. Heritability of clinical chemistries in an older twin cohort: the NHLBI Twin Study. Genet Epidemiol 1987;4:1-11. [Crossref] [PubMed]
- Makkonen J, Pietiläinen KH, Rissanen A, et al. Genetic factors contribute to variation in serum alanine aminotransferase activity independent of obesity and alcohol: a study in monozygotic and dizygotic twins. J Hepatol 2009;50:1035-42. [Crossref] [PubMed]
- Chen VL, Du X, Chen Y, et al. Genome-wide association study of serum liver enzymes implicates diverse metabolic and liver pathology. Nat Commun 2021;12:816. [Crossref] [PubMed]
- Wieme RJ, Demeulenaere L. Enzyme assays in liver disease. J Clin Pathol Suppl (Assoc Clin Pathol) 1970;4:51-9. [Crossref] [PubMed]
- Rej R. Aminotransferases in disease. Clin Lab Med 1989;9:667-87. [Crossref] [PubMed]
- Kobayashi A, Suzuki Y, Sugai S. Specificity of transaminase activities in the prediction of drug-induced hepatotoxicity. J Toxicol Sci 2020;45:515-37. [Crossref] [PubMed]
- Agius L, Tosh D. Acinar zonation of cytosolic but not organelle-bound activities of phosphoenolpyruvate carboxykinase and aspartate aminotransferase in guinea-pig liver. Biochem J 1990;271:387-91. [Crossref] [PubMed]
- Feilleux-Duché S, Garlatti M, Burcelin R, et al. Acinar zonation of the hormonal regulation of cytosolic aspartate aminotransferase in the liver. Am J Physiol 1994;266:C911-8. [Crossref] [PubMed]
- Cunningham RP, Porat-Shliom N. Liver Zonation - Revisiting Old Questions With New Technologies. Front Physiol 2021;12:732929. [Crossref] [PubMed]
- Braeuning A, Ittrich C, Köhle C, et al. Differential gene expression in periportal and perivenous mouse hepatocytes. FEBS J 2006;273:5051-61. [Crossref] [PubMed]
- Dufour DR, Lott JA, Nolte FS, et al. Diagnosis and monitoring of hepatic injury. I. Performance characteristics of laboratory tests. Clin Chem 2000;46:2027-49. [Crossref] [PubMed]
- McGill MR. The past and present of serum aminotransferases and the future of liver injury biomarkers. EXCLI J 2016;15:817-28. [PubMed]
- Nalpas B, Vassault A, Charpin S, et al. Serum mitochondrial aspartate aminotransferase as a marker of chronic alcoholism: diagnostic value and interpretation in a liver unit. Hepatology 1986;6:608-14. [Crossref] [PubMed]
- Nyblom H, Berggren U, Balldin J, et al. High AST/ALT ratio may indicate advanced alcoholic liver disease rather than heavy drinking. Alcohol Alcohol 2004;39:336-9. [Crossref] [PubMed]
- Kamimoto Y, Horiuchi S, Tanase S, et al. Plasma clearance of intravenously injected aspartate aminotransferase isozymes: evidence for preferential uptake by sinusoidal liver cells. Hepatology 1985;5:367-75. [Crossref] [PubMed]
- Price C, Alberti K. Biochemical assessment of liver function. In: Wright R, Albert KGMM, Karran S, et al. editors. Liver and biliary diseases—pathophysiology, diagnosis, management. London: WB Saunders, 1979:381-416.
- Córdoba J, O'Riordan K, Dupuis J, et al. Diurnal variation of serum alanine transaminase activity in chronic liver disease. Hepatology 1998;28:1724-5. [Crossref] [PubMed]
- Fraser CG. Biological variation in clinical chemistry: an update: collated data, 1988-1991. Arch Pathol Lab Med 1992;116:916-23. [PubMed]
- Pettersson J, Hindorf U, Persson P, et al. Muscular exercise can cause highly pathological liver function tests in healthy men. Br J Clin Pharmacol 2008;65:253-9. [Crossref] [PubMed]
- Kechagias S, Ernersson A, Dahlqvist O, et al. Fast-food-based hyper-alimentation can induce rapid and profound elevation of serum alanine aminotransferase in healthy subjects. Gut 2008;57:649-54. [Crossref] [PubMed]
- Ilan Y, Martinowitz G, Abramsky O, et al. Prolonged sleep-deprivation induced disturbed liver functions serum lipid levels, and hyperphosphatemia. Eur J Clin Invest 1992;22:740-3. [Crossref] [PubMed]
- Ozaki E, Ochiai H, Shirasawa T, et al. Eating quickly is associated with a low aspartate aminotransferase to alanine aminotransferase ratio in middle-aged adults: a large-scale cross-sectional survey in Japan. Arch Public Health 2020;78:101. [Crossref] [PubMed]
- Narjes H, Nehmiz G. Effect of hospitalisation on liver enzymes in healthy subjects. Eur J Clin Pharmacol 2000;56:329-33. [Crossref] [PubMed]
- Malakouti M, Kataria A, Ali SK, et al. Elevated Liver Enzymes in Asymptomatic Patients - What Should I Do? J Clin Transl Hepatol 2017;5:394-403. [Crossref] [PubMed]
- Kwo PY, Cohen SM, Lim JK. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. Am J Gastroenterol 2017;112:18-35. [Crossref] [PubMed]
- Mera JR, Dickson B, Feldman M. Influence of gender on the ratio of serum aspartate aminotransferase (AST) to alanine aminotransferase (ALT) in patients with and without hyperbilirubinemia. Dig Dis Sci 2008;53:799-802. [Crossref] [PubMed]
- Yasuda K, Okuda K, Endo N, et al. Hypoaminotransferasemia in patients undergoing long-term hemodialysis: clinical and biochemical appraisal. Gastroenterology 1995;109:1295-300. [Crossref] [PubMed]
- Claus M, Antoni C, Hofmann B. Factors associated with elevated alanine aminotransferase in employees of a German chemical company: results of a large cross-sectional study. BMC Gastroenterol 2021;21:25. [Crossref] [PubMed]
- Nunez DJ, Alexander M, Yerges-Armstrong L, et al. Factors influencing longitudinal changes of circulating liver enzyme concentrations in subjects randomized to placebo in four clinical trials. Am J Physiol Gastrointest Liver Physiol 2019;316:G372-86. [Crossref] [PubMed]
- Dong MH, Bettencourt R, Brenner DA, et al. Serum levels of alanine aminotransferase decrease with age in longitudinal analysis. Clin Gastroenterol Hepatol 2012;10:285-90.e1. [Crossref] [PubMed]
- Siest G, Schiele F, Galteau MM, et al. Aspartate aminotransferase and alanine aminotransferase activities in plasma: statistical distributions, individual variations, and reference values. Clin Chem 1975;21:1077-87. [Crossref] [PubMed]
- Qu HQ, Li Q, Grove ML, et al. Population-based risk factors for elevated alanine aminotransferase in a South Texas Mexican-American population. Arch Med Res 2012;43:482-8. [Crossref] [PubMed]
- Vajro P, Lofrano MM, Fontanella A, et al. Immunoglobulin complexed AST ("macro-AST") in an asymptomatic child with persistent hypertransaminasemia. J Pediatr Gastroenterol Nutr 1992;15:458-60. [Crossref] [PubMed]
- Whitehead TP, Robinson D, Allaway SL. The effects of cigarette smoking and alcohol consumption on serum liver enzyme activities: a dose-related study in men. Ann Clin Biochem 1996;33:530-5. [Crossref] [PubMed]
- Wannamethee SG, Shaper AG. Cigarette smoking and serum liver enzymes: the role of alcohol and inflammation. Ann Clin Biochem 2010;47:321-6. [Crossref] [PubMed]
- Ruhl CE, Everhart JE. Coffee and caffeine consumption reduce the risk of elevated serum alanine aminotransferase activity in the United States. Gastroenterology 2005;128:24-32. [Crossref] [PubMed]
- Horiuchi S, Kamimoto Y, Morino Y. Hepatic clearance of rat liver aspartate aminotransferase isozymes: evidence for endocytotic uptake via different binding sites on sinusoidal liver cells. Hepatology 1985;5:376-82. [Crossref] [PubMed]
- Schindhelm RK, Diamant M, Dekker JM, et al. Alanine aminotransferase as a marker of non-alcoholic fatty liver disease in relation to type 2 diabetes mellitus and cardiovascular disease. Diabetes Metab Res Rev 2006;22:437-43. [Crossref] [PubMed]
- Sorbi D, Boynton J, Lindor KD. The ratio of aspartate aminotransferase to alanine aminotransferase: potential value in differentiating nonalcoholic steatohepatitis from alcoholic liver disease. Am J Gastroenterol 1999;94:1018-22. [Crossref] [PubMed]
- Noureddin N, Noureddin M, Singh A, et al. Progression of Nonalcoholic Fatty Liver Disease-Associated Fibrosis in a Large Cohort of Patients with Type 2 Diabetes. Dig Dis Sci 2022;67:1379-88. [Crossref] [PubMed]
- Hall P, Cash J. What is the Real Function of the Liver ‘Function’ Tests? Ulster Med J 2012;81:30-6. [PubMed]
- Labayen I, Ruiz JR, Huybrechts I, et al. Ideal cardiovascular health and liver enzyme levels in European adolescents; the HELENA study. J Physiol Biochem 2017;73:225-34. [Crossref] [PubMed]
- Williams AL, Hoofnagle JH. Ratio of serum aspartate to alanine aminotransferase in chronic hepatitis. Relationship to cirrhosis. Gastroenterology 1988;95:734-9. [Crossref] [PubMed]
- Giannini E, Botta F, Fasoli A, et al. Progressive liver functional impairment is associated with an increase in AST/ALT ratio. Dig Dis Sci 1999;44:1249-53. [Crossref] [PubMed]
- Valenti L, Pelusi S, Bianco C, et al. Definition of Healthy Ranges for Alanine Aminotransferase Levels: A 2021 Update. Hepatol Commun 2021;5:1824-32. [Crossref] [PubMed]
- Xie K, Chen CH, Tsai SP, et al. Loss of Life Expectancy by 10 Years or More From Elevated Aspartate Aminotransferase: Finding Aspartate Aminotransferase a Better Mortality Predictor for All-Cause and Liver-Related than Alanine Aminotransferase. Am J Gastroenterol 2019;114:1478-87. [Crossref] [PubMed]
- Rej R. Multiple Molecular-Forms of Human Cytoplasmic Aspartate-Aminotransferase. Clin Chim Acta 1981;112:1-11. [Crossref] [PubMed]
- McGovern AJ, Vitkovitsky IV, Jones DL, et al. Can AST/ALT ratio indicate recovery after acute paracetamol poisoning? Clin Toxicol (Phila) 2015;53:164-7. [Crossref] [PubMed]
- Hofmeister MG, Xing J, Foster MA, et al. Factors Associated With Hepatitis A Mortality During Person-to-Person Outbreaks: A Matched Case-Control Study-United States, 2016-2019. Hepatology 2021;74:28-40. [Crossref] [PubMed]
- Ndrepepa G, Holdenrieder S, Kastrati A. Prognostic value of De Ritis ratio with aspartate aminotransferase and alanine aminotransferase within the reference range. Clin Chim Acta 2022; Epub ahead of print. [Crossref] [PubMed]
- Pinnaduwage L, Ye C, Hanley AJ, et al. Changes Over Time in Hepatic Markers Predict Changes in Insulin Sensitivity, β-Cell Function, and Glycemia. J Clin Endocrinol Metab 2018;103:2651-9. [Crossref] [PubMed]
- Weng SF, Kai J, Guha IN, et al. The value of aspartate aminotransferase and alanine aminotransferase in cardiovascular disease risk assessment. Open Heart 2015;2:e000272. [Crossref] [PubMed]
- Nakajima K, Yuno M, Tanaka K, et al. High Aspartate Aminotransferase/Alanine Aminotransferase Ratio May Be Associated with All-Cause Mortality in the Elderly: A Retrospective Cohort Study Using Artificial Intelligence and Conventional Analysis. Healthcare (Basel) 2022;10:674. [Crossref] [PubMed]
- Yokoyama M, Watanabe T, Otaki Y, et al. Association of the Aspartate Aminotransferase to Alanine Aminotransferase Ratio with BNP Level and Cardiovascular Mortality in the General Population: The Yamagata Study 10-Year Follow-Up. Dis Markers 2016;2016:4857917. [Crossref] [PubMed]
- Katzke V, Johnson T, Sookthai D, et al. Circulating liver enzymes and risks of chronic diseases and mortality in the prospective EPIC-Heidelberg case-cohort study. BMJ Open 2020;10:e033532. [Crossref] [PubMed]
- Liu H, Ding C, Hu L, et al. The association between AST/ALT ratio and all-cause and cardiovascular mortality in patients with hypertension. Medicine (Baltimore) 2021;100:e26693. [Crossref] [PubMed]
- Ferrannini G, Rosenthal N, Hansen MK, et al. Liver function markers predict cardiovascular and renal outcomes in the CANVAS Program. Cardiovasc Diabetol 2022;21:127. [Crossref] [PubMed]
- Liu H, Cao Y, Jin J, et al. Liver Fibrosis Scoring Systems as Novel Tools for Predicting Recurrent Cardiovascular Events in Patients with a Prior Cardiovascular Event. Cardiol Discov 2021;1:214-22. [Crossref]
- Alexander KS, Zakai NA, Lidofsky SD, et al. Non-alcoholic fatty liver disease, liver biomarkers and stroke risk: The Reasons for Geographic and Racial Differences in Stroke cohort. PLoS One 2018;13:e0194153. [Crossref] [PubMed]
- Liu H, Zha X, Ding C, et al. AST/ALT Ratio and Peripheral Artery Disease in a Chinese Hypertensive Population: A Cross-Sectional Study. Angiology 2021;72:916-22. [Crossref] [PubMed]
- Rief P, Pichler M, Raggam R, et al. The AST/ALT (De-Ritis) ratio: A novel marker for critical limb ischemia in peripheral arterial occlusive disease patients. Medicine (Baltimore) 2016;95:e3843. [Crossref] [PubMed]
- Liu HH, Cao YX, Jin JL, et al. Liver Fibrosis Scoring Systems as Novel Tools for Predicting Cardiovascular Outcomes in Patients Following Elective Percutaneous Coronary Intervention. J Am Heart Assoc 2021;10:e018869. [Crossref] [PubMed]
- Ndrepepa G, Holdenrieder S, Kastrati A. De Ritis ratio and long-term major cardiovascular adverse events in patients undergoing elective percutaneous coronary intervention. Eur J Clin Invest 2023; [Crossref] [PubMed]
- Djakpo DK, Wang ZQ, Shrestha M. The significance of transaminase ratio (AST/ALT) in acute myocardial infarction. Arch Med Sci Atheroscler Dis 2020;5:e279-83. [Crossref] [PubMed]
- Jasiewicz M, Siedlaczek M, Kasprzak M, et al. Elevated serum transaminases in patients with acute coronary syndromes: Do we need a revision of exclusion criteria for clinical trials? Cardiol J 2021; Epub ahead of print. [Crossref] [PubMed]
- Xu Q, Zhang X, Li H, et al. Aspartate aminotransferase to alanine aminotransferase ratio and clinical outcomes after acute ischemic stroke: the CNSR-III registry. Intern Emerg Med 2022;17:1987-96. [Crossref] [PubMed]
- Feng X, Wen Y, Peng FF, et al. Association between aminotransferase/alanine aminotransferase ratio and cardiovascular disease mortality in patients on peritoneal dialysis: a multi-center retrospective study. BMC Nephrol 2020;21:209. [Crossref] [PubMed]
- Nam JS, Kim WJ, An SM, et al. Age-dependent relationship between preoperative serum aminotransferase and mortality after cardiovascular surgery. Aging (Albany NY) 2019;11:9060-74. [Crossref] [PubMed]
- Yin M, Zhang H, Liu Q, et al. Diagnostic Performance of Clinical Laboratory Indicators With Sarcopenia: Results From the West China Health and Aging Trend Study. Front Endocrinol (Lausanne) 2021;12:785045. [Crossref] [PubMed]
- He Y, Ding F, Yin M, et al. High Serum AST/ALT Ratio and Low Serum INS*PA Product Are Risk Factors and Can Diagnose Sarcopenia in Middle-Aged and Older Adults. Front Endocrinol (Lausanne) 2022;13:843610. [Crossref] [PubMed]
- Maeda D, Sakane K, Kanzaki Y, et al. Relation of Aspartate Aminotransferase to Alanine Aminotransferase Ratio to Nutritional Status and Prognosis in Patients With Acute Heart Failure. Am J Cardiol 2021;139:64-70. [Crossref] [PubMed]
- Jo KM, Heo NY, Park SH, et al. Serum Aminotransferase Level in Rhabdomyolysis according to Concurrent Liver Disease. Korean J Gastroenterol 2019;74:205-11. [Crossref] [PubMed]
- Wang J, Li J, Ren Y, et al. Association between Alanine Aminotransferase/Aspartate Aminotransferase Ratio (AST/ALT Ratio) and Coronary Artery Injury in Children with Kawasaki Disease. Cardiol Res Pract 2020;2020:8743548. [Crossref] [PubMed]
- Iacobellis G, Pellicelli AM, Grisorio B, et al. Relation of epicardial fat and alanine aminotransferase in subjects with increased visceral fat. Obesity (Silver Spring) 2008;16:179-83. [Crossref] [PubMed]
- He HM, He C, You ZB, et al. Non-Invasive Liver Fibrosis Scores Are Associated With Contrast-Associated Acute Kidney Injury in Patients Undergoing Elective Percutaneous Coronary Intervention. Angiology 2022; Epub ahead of print. [Crossref] [PubMed]
- Pilarczyk K, Carstens H, Papathanasiou M, et al. Prediction of acute kidney injury after left ventricular assist device implantation: Evaluation of clinical risk scores. Artif Organs 2020;44:162-73. [Crossref] [PubMed]
- Schettle S, Rosenbaum A, Goodman D, et al. De Ritis-adjusted AST provides comparable efficacy to lactate dehydrogenase as a biomarker for detection of LVAD hemolysis or thrombosis. Artif Organs 2022;46:1425-8. [Crossref] [PubMed]
- Pilarczyk K, Carstens H, Heckmann J, et al. The aspartate transaminase/alanine transaminase (DeRitis) ratio predicts mid-term mortality and renal and respiratory dysfunction after left ventricular assist device implantation. Eur J Cardiothorac Surg 2017;52:781-8. [Crossref] [PubMed]
- Gultekin Y, Bolat A, Hatice K, et al. Does Aspartate Aminotransferase to Alanine Aminotransferase Ratio Predict Acute Kidney Injury After Cardiac Surgery? Heart Surg Forum 2021;24:E506-11. [Crossref] [PubMed]
- Ewid M, Sherif H, Allihimy AS, et al. AST/ALT ratio predicts the functional severity of chronic heart failure with reduced left ventricular ejection fraction. BMC Res Notes 2020;13:178. [Crossref] [PubMed]
- Wang L, Xu Y, Zhang S, et al. The AST/ALT Ratio (De Ritis Ratio) Represents an Unfavorable Prognosis in Patients in Early-Stage SFTS: An Observational Cohort Study. Front Cell Infect Microbiol 2022;12:725642. [Crossref] [PubMed]
- Liu Y, Zhao P, Cheng M, et al. AST to ALT ratio and arterial stiffness in non-fatty liver Japanese population:a secondary analysis based on a cross-sectional study. Lipids Health Dis 2018;17:275. [Crossref] [PubMed]
- Yildirim A, Abacioglu OO, Koyunsever NY. The relationship between microvascular angina and De Ritis ratio in normal coronary artery patients with recurrent chest pain / De Ritis ratio and microvascular angina. Dicle Med J 2020;47:846-51. [Crossref]
- Akbuğa K, Yayla KG, Yayla Ç. Evaluation of the relationship between aspartate aminotransferase/alanine aminotransferase ratio and coronary slow-flow phenomenon. Biomark Med 2022;16:783-9. [Crossref] [PubMed]
- Aksoy MNM, Turna F, Sahin I, et al. Is AST/ALT Ratio a Predictor of In-hospital Mortality in Pulmonary Embolism Patients? J Coll Physicians Surg Pak 2022;32:171-6. [Crossref] [PubMed]
- Yogeswaran A, Tello K, Lund J, et al. Risk assessment in pulmonary hypertension based on routinely measured laboratory parameters. J Heart Lung Transplant 2022;41:400-10. [Crossref] [PubMed]
- Su WT, Rau CS, Chou SE, et al. Association between Elevated De Ritis Ratio and Mortality Outcome in Adult Patients with Thoracoabdominal Trauma. Healthcare (Basel) 2022;10:2082. [Crossref] [PubMed]
- Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1789-858. [Crossref] [PubMed]
- Baker S, Chambers C, McQuillan P, et al. Myocardial perfusion imaging is an effective screening test for coronary artery disease in liver transplant candidates. Clin Transplant 2015;29:319-26. [Crossref] [PubMed]
- Patel SS, Nabi E, Guzman L, et al. Coronary artery disease in decompensated patients undergoing liver transplantation evaluation. Liver Transpl 2018;24:333-42. [Crossref] [PubMed]
- Edens MA, Kuipers F, Stolk RP. Non-alcoholic fatty liver disease is associated with cardiovascular disease risk markers. Obes Rev 2009;10:412-9. [Crossref] [PubMed]
- Kadayifci A, Tan V, Ursell PC, et al. Clinical and pathologic risk factors for atherosclerosis in cirrhosis: a comparison between NASH-related cirrhosis and cirrhosis due to other aetiologies. J Hepatol 2008;49:595-9. [Crossref] [PubMed]
- Singh V, Patel NJ, Rodriguez AP, et al. Percutaneous Coronary Intervention in Patients With End-Stage Liver Disease. Am J Cardiol 2016;117:1729-34. [Crossref] [PubMed]
- Istanbuly S, Matetic A, Mohamed MO, et al. Comparison of Outcomes of Patients With Versus Without Chronic Liver Disease Undergoing Percutaneous Coronary Intervention. Am J Cardiol 2021;156:32-8. [Crossref] [PubMed]
- Alqahtani F, Balla S, AlHajji M, et al. Temporal trends in the utilization and outcomes of percutaneous coronary interventions in patients with liver cirrhosis. Catheter Cardiovasc Interv 2020;96:802-10. [Crossref] [PubMed]
- Matetic A, Contractor T, Mohamed MO, et al. Trends, management and outcomes of acute myocardial infarction in chronic liver disease. Int J Clin Pract 2021;75:e13841. [Crossref] [PubMed]
- The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2020;5:245-66. [Crossref] [PubMed]
- Riazi K, Azhari H, Charette JH, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2022;7:851-61. [Crossref] [PubMed]
- Bacon BR, Farahvash MJ, Janney CG, et al. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology 1994;107:1103-9. [Crossref] [PubMed]
- Ruhl CE, Everhart JE. Joint effects of body weight and alcohol on elevated serum alanine aminotransferase in the United States population. Clin Gastroenterol Hepatol 2005;3:1260-8. [Crossref] [PubMed]
- Angulo P, Keach JC, Batts KP, et al. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology 1999;30:1356-62. [Crossref] [PubMed]
- Osna NA, Donohue TM Jr, Kharbanda KK. Alcoholic Liver Disease: Pathogenesis and Current Management. Alcohol Res 2017;38:147-61. [PubMed]
- Diehl AM, Potter J, Boitnott J, et al. Relationship between pyridoxal 5'-phosphate deficiency and aminotransferase levels in alcoholic hepatitis. Gastroenterology 1984;86:632-6. [Crossref] [PubMed]
- Pol S, Nalpas B, Vassault A, et al. Hepatic activity and mRNA expression of aspartate aminotransferase isoenzymes in alcoholic and nonalcoholic liver disease. Hepatology 1991;14:620-5. [PubMed]
- Piano MR. Alcohol's Effects on the Cardiovascular System. Alcohol Res 2017;38:219-41. [PubMed]
- Larsson SC, Burgess S, Mason AM, et al. Alcohol Consumption and Cardiovascular Disease: A Mendelian Randomization Study. Circ Genom Precis Med 2020;13:e002814. [Crossref] [PubMed]
- Ladue JS, Wroblewski F, Karmen A. Serum glutamic oxaloacetic transaminase activity in human acute transmural myocardial infarction. Science 1954;120:497-9. [Crossref] [PubMed]
- Lazzeri C, Valente S, Boddi M, et al. Clinical and prognostic significance of increased liver enzymes in ST-elevation myocardial infarction. Int J Cardiol 2014;177:543-4. [Crossref] [PubMed]
- Lofthus DM, Stevens SR, Armstrong PW, et al. Pattern of liver enzyme elevations in acute ST-elevation myocardial infarction. Coron Artery Dis 2012;23:22-30. [Crossref] [PubMed]
- Gao M, Cheng Y, Zheng Y, et al. Association of serum transaminases with short- and long-term outcomes in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. BMC Cardiovasc Disord 2017;17:43. [Crossref] [PubMed]
- Li J, Zhao Z, Jiang H, et al. Predictive value of elevated alanine aminotransferase for in-hospital mortality in patients with acute myocardial infarction. BMC Cardiovasc Disord 2021;21:82. [Crossref] [PubMed]
- Moon J, Kang W, Oh PC, et al. Serum transaminase determined in the emergency room predicts outcomes in patients with acute ST-segment elevation myocardial infarction who undergo primary percutaneous coronary intervention. Int J Cardiol 2014;177:442-7. [Crossref] [PubMed]
- Naschitz JE, Slobodin G, Lewis RJ, et al. Heart diseases affecting the liver and liver diseases affecting the heart. Am Heart J 2000;140:111-20. [Crossref] [PubMed]
- Seeto RK, Fenn B, Rockey DC. Ischemic hepatitis: clinical presentation and pathogenesis. Am J Med 2000;109:109-13. [Crossref] [PubMed]
- Henrion J. Hypoxic hepatitis. Liver Int 2012;32:1039-52. [Crossref] [PubMed]
- Schupp T, Weidner K, Rusnak J, et al. Diagnostic and prognostic value of the AST/ALT ratio in patients with sepsis and septic shock. Scand J Gastroenterol 2022; Epub ahead of print. [Crossref] [PubMed]
- Li R, Zhu WJ, Wang F, et al. AST/ALT ratio as a predictor of mortality and exacerbations of PM/DM-ILD in 1 year-a retrospective cohort study with 522 cases. Arthritis Res Ther 2020;22:202. [Crossref] [PubMed]
- Le Couteur DG, Blyth FM, Creasey HM, et al. The association of alanine transaminase with aging, frailty, and mortality. J Gerontol A Biol Sci Med Sci 2010;65:712-7. [Crossref] [PubMed]
- Elinav E, Ackerman Z, Maaravi Y, et al. Low alanine aminotransferase activity in older people is associated with greater long-term mortality. J Am Geriatr Soc 2006;54:1719-24. [Crossref] [PubMed]
- Ford I, Mooijaart SP, Lloyd S, et al. The inverse relationship between alanine aminotransferase in the normal range and adverse cardiovascular and non-cardiovascular outcomes. Int J Epidemiol 2011;40:1530-8. [Crossref] [PubMed]
- Elinav E, Ben-Dov IZ, Ackerman E, et al. Correlation between serum alanine aminotransferase activity and age: an inverted U curve pattern. Am J Gastroenterol 2005;100:2201-4. [Crossref] [PubMed]
- Yamazaki H, Kamitani T, Matsui T, et al. Association of low alanine aminotransferase with loss of independence or death: A 5-year population-based cohort study. J Gastroenterol Hepatol 2019;34:1793-9. [Crossref] [PubMed]
- Ruhl CE, Everhart JE. The association of low serum alanine aminotransferase activity with mortality in the US population. Am J Epidemiol 2013;178:1702-11. [Crossref] [PubMed]
- Vespasiani-Gentilucci U, De Vincentis A, Ferrucci L, et al. Low Alanine Aminotransferase Levels in the Elderly Population: Frailty, Disability, Sarcopenia, and Reduced Survival. J Gerontol A Biol Sci Med Sci 2018;73:925-30. [Crossref] [PubMed]
- Hyde Z, Flicker L, Almeida OP, et al. Low free testosterone predicts frailty in older men: the health in men study. J Clin Endocrinol Metab 2010;95:3165-72. [Crossref] [PubMed]
- Farias JM, Tinetti M, Khoury M, et al. Low testosterone concentration and atherosclerotic disease markers in male patients with type 2 diabetes. J Clin Endocrinol Metab 2014;99:4698-703. [Crossref] [PubMed]
- Malkin CJ, Pugh PJ, Morris PD, et al. Low serum testosterone and increased mortality in men with coronary heart disease. Heart 2010;96:1821-5. [Crossref] [PubMed]
- Lumeng L. The role of acetaldehyde in mediating the deleterious effect of ethanol on pyridoxal 5'-phosphate metabolism. J Clin Invest 1978;62:286-93. [Crossref] [PubMed]
- Loohuis LM, Albersen M, de Jong S, et al. The Alkaline Phosphatase (ALPL) Locus Is Associated with B6 Vitamer Levels in CSF and Plasma. Genes (Basel) 2018.
- Sutoh Y, Hachiya T, Suzuki Y, et al. ALDH2 genotype modulates the association between alcohol consumption and AST/ALT ratio among middle-aged Japanese men: a genome-wide G × E interaction analysis. Sci Rep 2020;10:16227. [Crossref] [PubMed]
- Lindena J, Sommerfeld U, Höpfel C, et al. Catalytic enzyme activity concentration in tissues of man, dog, rabbit, guinea pig, rat and mouse. Approach to a quantitative diagnostic enzymology, III. Communication. J Clin Chem Clin Biochem 1986;24:35-47. [Crossref] [PubMed]
- Porter SA, Pedley A, Massaro JM, et al. Aminotransferase levels are associated with cardiometabolic risk above and beyond visceral fat and insulin resistance: the Framingham Heart Study. Arterioscler Thromb Vasc Biol 2013;33:139-46. [Crossref] [PubMed]
- Goessling W, Massaro JM, Vasan RS, et al. Aminotransferase levels and 20-year risk of metabolic syndrome, diabetes, and cardiovascular disease. Gastroenterology 2008;135:1935-44, 1944.e1.
- Monami M, Bardini G, Lamanna C, et al. Liver enzymes and risk of diabetes and cardiovascular disease: results of the Firenze Bagno a Ripoli (FIBAR) study. Metabolism 2008;57:387-92. [Crossref] [PubMed]
- Kim HR, Han MA. Association between Serum Liver Enzymes and Metabolic Syndrome in Korean Adults. Int J Environ Res Public Health 2018; [Crossref] [PubMed]
- Greber-Platzer S, Thajer A, Bohn S, et al. Increased liver echogenicity and liver enzymes are associated with extreme obesity, adolescent age and male gender: analysis from the German/Austrian/Swiss obesity registry APV. BMC Pediatr 2019;19:332. [Crossref] [PubMed]
- Sette LH, Lopes EP. The reduction of serum aminotransferase levels is proportional to the decline of the glomerular filtration rate in patients with chronic kidney disease. Clinics (Sao Paulo) 2015;70:346-9. [Crossref] [PubMed]
- Ono K, Ono T, Matsumata T. The pathogenesis of decreased aspartate aminotransferase and alanine aminotransferase activity in the plasma of hemodialysis patients: the role of vitamin B6 deficiency. Clin Nephrol 1995;43:405-8. [PubMed]
- Huang JW, Yen CJ, Pai MF, et al. Association between serum aspartate transaminase and homocysteine levels in hemodialysis patients. Am J Kidney Dis 2002;40:1195-201. [Crossref] [PubMed]
- Lopes EP, Sette LH, Sette JB, et al. Serum alanine aminotransferase levels, hematocrit rate and body weight correlations before and after hemodialysis session. Clinics (Sao Paulo) 2009;64:941-5. [Crossref] [PubMed]
- Ray L, Nanda SK, Chatterjee A, et al. A comparative study of serum aminotransferases in chronic kidney disease with and without end-stage renal disease: Need for new reference ranges. Int J Appl Basic Med Res 2015;5:31-5. [Crossref] [PubMed]
- Hanley AJ, Williams K, Festa A, et al. Liver markers and development of the metabolic syndrome: the insulin resistance atherosclerosis study. Diabetes 2005;54:3140-7. [Crossref] [PubMed]
- Yadav D, Choi E, Ahn SV, et al. Incremental Predictive Value of Serum AST-to-ALT Ratio for Incident Metabolic Syndrome: The ARIRANG Study. PLoS One 2016;11:e0161304. [Crossref] [PubMed]
- Lin S, Tang L, Jiang R, et al. The Relationship Between Aspartate Aminotransferase To Alanine Aminotransferase Ratio And Metabolic Syndrome In Adolescents In Northeast China. Diabetes Metab Syndr Obes 2019;12:2387-94. [Crossref] [PubMed]
- Nakajima H, Okada H, Hamaguchi M, et al. Low aspartate aminotransferase/alanine aminotransferase ratio is a predictor of diabetes incidence in Japanese people: Population-based Panasonic cohort study 5. Diabetes Metab Res Rev 2022;38:e3553. [Crossref] [PubMed]
- Niu H, Zhou Y. Nonlinear Relationship Between AST-to-ALT Ratio and the Incidence of Type 2 Diabetes Mellitus: A Follow-Up Study. Int J Gen Med 2021;14:8373-82. [Crossref] [PubMed]
- Chen L, Zhang K, Li X, et al. Association Between Aspartate Aminotransferase to Alanine Aminotransferase Ratio and Incidence of Type 2 Diabetes Mellitus in the Japanese Population: A Secondary Analysis of a Retrospective Cohort Study. Diabetes Metab Syndr Obes 2021;14:4483-95. [Crossref] [PubMed]
- Ueland PM, Ulvik A, Rios-Avila L, et al. Direct and Functional Biomarkers of Vitamin B6 Status. Annu Rev Nutr 2015;35:33-70. [Crossref] [PubMed]
- Kotronen A, Yki-Järvinen H, Sevastianova K, et al. Comparison of the relative contributions of intra-abdominal and liver fat to components of the metabolic syndrome. Obesity (Silver Spring) 2011;19:23-8. [Crossref] [PubMed]
- Westerbacka J, Cornér A, Tiikkainen M, et al. Women and men have similar amounts of liver and intra-abdominal fat, despite more subcutaneous fat in women: implications for sex differences in markers of cardiovascular risk. Diabetologia 2004;47:1360-9. [Crossref] [PubMed]
- Bonnet F, Ducluzeau PH, Gastaldelli A, et al. Liver enzymes are associated with hepatic insulin resistance, insulin secretion, and glucagon concentration in healthy men and women. Diabetes 2011;60:1660-7. [Crossref] [PubMed]
- Ruhl CE, Everhart JE. Elevated serum alanine aminotransferase and gamma-glutamyltransferase and mortality in the United States population. Gastroenterology 2009;136:477-85.e11. [Crossref] [PubMed]
- Siddiqui MS, Sterling RK, Luketic VA, et al. Association between high-normal levels of alanine aminotransferase and risk factors for atherogenesis. Gastroenterology 2013;145:1271-9.e1-3.
- Lorenzo C, Hanley AJ, Rewers MJ, et al. The association of alanine aminotransferase within the normal and mildly elevated range with lipoproteins and apolipoproteins: the Insulin Resistance Atherosclerosis Study. Diabetologia 2013;56:746-57. [Crossref] [PubMed]
- Kain K, Carter AM, Grant PJ, et al. Alanine aminotransferase is associated with atherothrombotic risk factors in a British South Asian population. J Thromb Haemost 2008;6:737-41. [Crossref] [PubMed]
- He KP, Zhao C, Qiang Y, et al. Impact of elevated aspartate and alanine aminotransferase on metabolic syndrome and its components among adult people living in Ningxia, China. Chronic Dis Transl Med 2015;1:124-32. [PubMed]
- Ioannou GN, Weiss NS, Boyko EJ, et al. Elevated serum alanine aminotransferase activity and calculated risk of coronary heart disease in the United States. Hepatology 2006;43:1145-51. [Crossref] [PubMed]
- Kim K, Kim DS, Kim KN. Serum Alanine Aminotransferase Level as a Risk Factor for Coronary Heart Disease Prediction in Koreans: Analysis of the Korea National Health and Nutrition Examination Survey (V-1, 2010 and V-2, 2011). Korean J Fam Med 2019;40:124-8. [Crossref] [PubMed]
- Schindhelm RK, Diamant M, Bakker SJ, et al. Liver alanine aminotransferase, insulin resistance and endothelial dysfunction in normotriglyceridaemic subjects with type 2 diabetes mellitus. Eur J Clin Invest 2005;35:369-74. [Crossref] [PubMed]
- Jung DH, Lee YJ, Ahn HY, et al. Relationship of hepatic steatosis and alanine aminotransferase with coronary calcification. Clin Chem Lab Med 2010;48:1829-34. [Crossref] [PubMed]
- Shen J, Zhang J, Wen J, et al. Correlation of serum alanine aminotransferase and aspartate aminotransferase with coronary heart disease. Int J Clin Exp Med 2015;8:4399-404. [PubMed]
- Masoudkabir F, Karbalai S, Vasheghani-Farahani A, et al. The association of liver transaminase activity with presence and severity of premature coronary artery disease. Angiology 2011;62:614-9. [Crossref] [PubMed]
- Saito T, Nishise Y, Makino N, et al. Impact of metabolic syndrome on elevated serum alanine aminotransferase levels in the Japanese population. Metabolism 2009;58:1067-75. [Crossref] [PubMed]
Cite this article as: Ndrepepa G. De Ritis ratio and cardiovascular disease: evidence and underlying mechanisms. J Lab Precis Med 2023;8:6.


