
Potential analytical interferences in cardiac troponin immunoassays
Introduction
Background
For routine laboratory diagnosis of myocardial injury, cardiac troponin I (cTnI) and cardiac troponin T (cTnT) are currently the most sensitive and cardiac-specific laboratory parameters (1-6). Due to the high analytical sensitivity of high-sensitivity (hs) cardiac troponin (cTn) routine assays, unexpectedly increased test results without an obvious clinical correlate are increasingly seen in daily clinical practice (4-6). In the majority of these patients, the cTn increase is caused by acute or chronic myocardial injury due to a variety of cardiac or primarily non-cardiac pathologies with cardiac involvement unrelated to an acute myocardial infarction (AMI). Sometimes in these patients, myocardial injury may not be detected by imaging as well (3-6). The numerous different mechanisms of myocardial injury have been comprehensively reviewed previously (1-6), and the most important diseases causing myocardial injury are summarized in Table 1. They mainly comprise of myocardial ischemia caused by an acute coronary syndrome (i.e., coronary plaque rupture or erosion with intracoronary thrombus formation; type 1 myocardial infarction), myocardial ischemia unrelated to an acute coronary syndrome (type 2 myocardial infarction), and myocardial injury unrelated to myocardial ischemia, such as inflammation (e.g., myocarditis), increased myocardial wall stress (e.g., in heart failure), toxic myocardial injury, and trauma (e.g., heart contusion).
Table 1
| Pathology | Cause |
|---|---|
| Type 1 AMI | Acute coronary syndromes (coronary plaque rupture or erosion with intracoronary thrombus formation) |
| Type 2 AMI | Cardiac oxygen supply/demand imbalance, e.g., by prolonged tachy- or bradyarrhythmias, coronary microvascular dysfunction, hypertensive urgency or crisis, hemorrhagic shock, acute respiratory failure, or non-atherothrombotic coronary causes (e.g., spontaneous dissection, vasospasm, embolism, or vasculitis) |
| Other causes of acute myocardial injury | E.g., acute heart failure, acute pulmonary embolism, acute myocarditis, Takotsubo cardiomyopathy, cardiac contusion, cardiac surgery, cardiac ablation therapy, frequent defibrillator shocks, cardiotoxic agents (e.g., drugs, chemotherapeutic agents), sepsis (multifactorial causes of myocardial injury), severe neurological diseases (e.g., hemorrhage, ischemic stroke, traumatic brain injury leading to massive central sympathetic activation), strenuous long lasting endurance exercise (e.g., marathon running) |
| Cardiac causes of chronic myocardial injury | E.g., chronic structural heart diseases, such as heart failure, chronic myocarditis, severe valvular heart diseases, cardiac amyloidosis |
| Primarily non-cardiac diseases with myocardial injury | E.g., pulmonary disease with severe hypoxia, chronic kidney diseases with severe renal failure, severe anemia, infiltrative diseases (e.g., myeloma with cardiac amyloidosis, systemic sarcoidosis with cardiac granulomas) |
Serial cardiac troponin testing is essential for differentiation of acute from chronic myocardial injury to detect significant changes (>20% from baseline values). AMI, acute myocardial infarction.
Rationale and knowledge gap
Clinicians, however, should be also aware to the fact, that no laboratory assay is perfect and outliers or rare analytical interferences may occasionally cause unexpectedly high or low cTn test results that do not match the clinical picture of the patient (6). Interferences are defined as all substances in blood samples that alter the correct value of a laboratory test result (e.g., cTn). Some patient populations [e.g., with a history of previous myocardial injury (e.g., myocarditis), autoimmune diseases, treatment with checkpoint inhibitors or monoclonal antibodies, of close contact with animals in particular mice, or with multiple myeloma] are theoretically more prone to antibody interferences, which are the most common form of analytical interferences. Apart from easily by visual inspection or by analyzers’ automatically calculated indices identifiable obvious potential interferences, such as severely icteric, hemolytic, lipemic samples, or clots, the feedback of clinicians is essential for laboratories to test for outliers and to identify other rare analytical interferences, such as antibodies. False-positive test results are usually more disturbing clinically and thereby easier identified in daily clinical practice. Patients with false low or even negative cTn values are also at risk for mistreatment or mismanagement, but they are less likely to be identified, because they are usually treated and managed correctly according to their clinical presentation. A recent review of 222 published case reports of cTn interference found, that 221 cases had false cTn elevations and only one case was found because of false low cTn concentrations (7). However, in patients with clinical and/or electrocardiographic or imaging evidence for acute myocardial injury with unexpectedly low or even negative cTn test results the laboratory should be contacted as well to test for analytical interferences.
Objective
This review is aimed to increase the awareness of clinicians of the fact that no laboratory assay is perfect and the possibility of outliers or rare analytical interferences in immunoassays leading to false cTn test results. The importance of a close collaboration of clinicians with the laboratory for the identification of such causes which are not obviously identified by inspecting the sample is stressed. We present an algorithm (see Figure 1) how to work-up unexpectedly increased cTn test results and summarize simple analytical methods which can be performed in routine clinical laboratories to identify the most common analytical interferences in cTn assays.
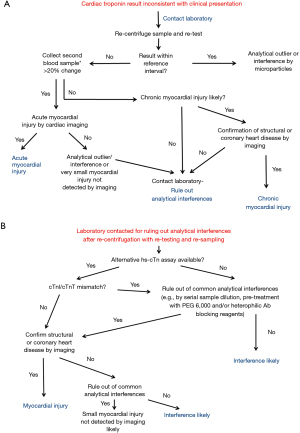
Work-up of questionable troponin test results
Current routine hs-cTn assays of all main manufacturers are optimized to reduce analytical confounders, and, therefore, analytical interferences are nowadays a rare cause for false hs-cTn test results (6-11). For example, usually F(ab')2 or Fab'region antibody fragments (removal of the Fc fragment) and so-called chimeric mouse-humanized antibodies (mouse variable/human constant region of Fab fragment) are frequently used in routine assays to reduce the potential of human anti-mouse antibodies or other heterophilic antibody interferences in patient samples. Recombinant single-chain variable fragment (scFv) antibodies also have great potential for this purpose (12). Additionally, heterophilic antibody blocking agents are added to assay reagents to reduce the risk for this kind of assay interference (e.g., by inclusion of aggregated blocking immunoglobulin of the same species as the assay antibodies or heterophilic antibody blocking antibodies in the assay buffers). Plasma is the matrix of choice because it reduces turnaround time by eliminating clotting time and avoids problems associated with prolonged clotting time in patients with prolonged coagulation (e.g., by treatment or liver failure).
However, clinicians should be aware of the fact that no immunoassay is perfect with the consequence of a small residual risk for false test results (6,7,13,14) that in part seems to be dependent on the cTn assay platform used and the tested patient population. Thus, clinicians should not accept laboratory results at face values without a possibility of error. In patients without cardiac symptoms, unnecessary and potentially costly or harmful cardiac investigations should be avoided unless indicated for reasons other than just an elevated cTn test result. When analytic interference with the cTn test is suspected clinically in stable, non-critically ill patients with a low probability of cardiac disease, the laboratory should be first contacted to rule out a so-called outliers or common analytic interferences. The latter usually requires additional blood sampling. As a first simplest step (see Figure 1A), cTn should be re-tested in the original blood sample after re-centrifugation to rule out outliers. Outliers are false, random, non-repeatable test results that are not due to analytical imprecision (15-17). The reported outlier rate for hs-cTn tests is low (approximately 0.3–0.5%) (15-17). The risk of misclassification of patients as false positive or negative due to outliers has been reported to be low (<0.3%) (7,17). A second blood sample has to be collected, if an outlier could be ruled out. This allows the most common causes of false test results, i.e., particles in the sample (e.g., fibrin micro clots), carry-over between samples within the analyzer, mix-up of samples, to be quickly excluded. In this newly collected blood sample and the baseline sample, ideally additionally the other cTn should be tested as well (see Figure 1B), if available in the laboratory (i.e., cTnI should be the back-up parameter if cTnT is routinely measured and vice versa) to quickly check for analytical interferences. Analytically interfering substances typically react differently in different analytical systems and with different analytes (e.g., in different cTnI and cTnT assays). Analytical interferences often manifest as stable, unchanging values over several days but they frequently also result in fluctuating values over a period of several weeks in clinical situations that do not fit to the cTn test result. A change of cTn concentrations >20% in the newly drawn blood sample from the first baseline sample >3 h apart usually indicates acute myocardial injury, but may sometimes also be due to analytical problems.
There are several possible interferences causing erroneous test results, such as the typical analytical interferences severe hemolysis, hyperlipidemia or hyperbilirubinemia that may disturb the assay signal. In contrast to previous assay generations based on e.g., photometric or nephelometric detection, in the concentration ranges usually seen clinically (assay dependent published lack of interference for total bilirubin up to 15–100 mg/dL and for triglycerides up to 1,000–15,000 mg/dL), they are not of particular concern with the chemiluminescence detection technology of current routine hs-cTn assays (8,10,11). However, they may be relevant with some assays at extreme concentrations, e.g., pronounced hemolysis (>1 g hemoglobin/L) has been reported to cause falsely low hs-cTnT values (11) and to a lesser extent falsely low hs-cTnI results with some susceptible cTnI assays (18). Therefore, the laboratory staff must be aware of the specific assay performance characteristics of their hs-cTn assay in use in case of questionable cTn test results, and no generally valid statements are possible.
Immunoassays based on the streptavidin-biotin technology are sensitive to interference from very high biotin concentrations in blood samples and the lab should be aware of this effect (19). Biotin may cause falsely low cTnT and cTnI test results in some cTnI-assays (18), but not in others (20). Especially in the USA, biotin, a water-soluble B-complex vitamin, has become a popular over-the-counter dietary supplement (typical content 5–10 mg) used, for example, to strengthen hair and nails or to alleviate peripheral neuropathy. The unintended consequence of its use is the potential for false-negative test results in sandwich immunoassay based on streptavidin-biotin technology. This interference with the hs-cTnT assay was first reported by U.S. laboratories (19), but it does not seem to be an important source of erroneous results in clinical practice, particularly in European populations (21,22). In addition, the hs-cTnT assay has been optimized to reduce this kind of interference (18).
Other very rare potential interferences: assays using alkaline phosphatase labelled detection antibodies to generate the signal are theoretically susceptible to interference with very high concentrations of circulating alkaline phosphatase in samples (e.g., in severe hepatic or bone diseases), which could yield false positive test results. With previous cTn assay generations this kind of interference has been occasionally reported (23). In contrast to immunoassays for detection of circulating hormones an influence of total protein or albumin concentrations on cTn test results or specific cTn binding proteins have not been reported so far (8-11). Although the cross-reactivities of cTn antibodies with their skeletal muscle troponin isoforms are very small, occasionally (e.g., in very severe rhabdomyolysis) this could be an issue at extreme skeletal muscle troponin concentrations (24). Antibodies specifically targeting assay reagents such as streptavidin or ruthenium have also been reported occasionally (25,26).
Typical antibody interferences
Heterophilic or human anti-mouse antibodies
Heterophilic antibodies are endogenous human antibodies that react with other antigens than its specific antigen and also bind to immunoglobulins of other species probably mediated via the Fc region (27,28). Thereby they may interfere assay-dependently with all immunoassays, particularly 2-site immunometric assays, which are used for hs-cTn determination (see Figures 2-4). The overall reported prevalence of heterophilic antibodies is in the range between 0.1% to 3.1% in the general population (29). The more widespread use of antibody fragments without the Fc region and aggregated immunoglobulin in immunoassays improved the resistance of routine assays to this kind of potential interference, but heterophilic antibodies remain a threat in immunometric cTn assays. Heterophilic antibodies consist of two different groups: (I) they may be polyvalent antibodies formed in response to contact with foreign animal proteins with low affinities (e.g., after vaccination, viral infections, contact with animals, blood transfusion), which generally interfere to a lesser extent in tests due to their low affinity; and (II) monovalent antibodies with high affinity, e.g., human anti-mouse antibodies after treatment with mouse monoclonal antibodies or after close contact with mice, with high immunoassay interference potential (27,28). Despite the use of mouse-human chimeric antibodies in immunoassays, very-high titres of specific human anti-mouse antibodies can lead to false test results. Occasionally, also very high titres of heterophilic antibodies may be present in individual blood samples (e.g., after recent viral infections), which are not sufficiently blocked by the heterophilic blocking reagent that is usually included in the immunoassay reagents (29-32). In a systematic review of the reported cases, they were the 2nd most frequent cause of false positive cTn test results (7).
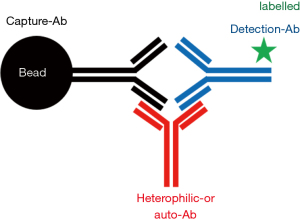
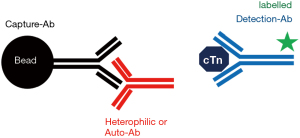
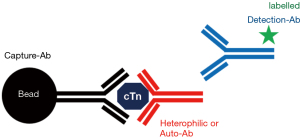
Dilution studies of the sample with the cTn assay diluent can be performed as a simple screening test to unmask antibody interferences, if a positive test result is sufficiently high (see Figure 5). In dilution studies, the protocol must be validated in control samples of a similar concentration (e.g., from a patient with myocardial infarction) to establish the expected recovery and to exclude matrix effects introduced by the diluent. This method has obvious limitations in terms of the precision of a cTn assay at its low measurement range, and each dilution must be measured at least in duplicate. In dilution studies (1:2, 1:4, 1:8, etc.), samples may not dilute linearly—as expected—if a confounding factor is present. If a test result is suspected to be falsely low, serial dilutions can alternatively be performed using a patient sample with a known high concentration. A significant deviation from linearity can be taken as evidence of the presence of an interfering substance, but apparent parallelism does not prove the absence of an interfering substance. All-in-all this simple screening method has limitations and can neither reliably rule out nor prove analytical interferences (e.g., macro-cTn is often missed) (29-34). This test has always to be followed by polyethylene glycol (PEG 6000) precipitation and testing for heterophilic antibodies, if PEG precipitation indicates interference by macromolecules or is inconclusive.
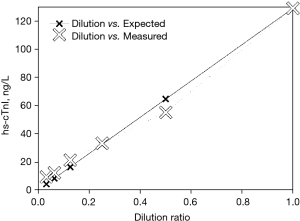
PEG pre-treatment of samples eliminates high molecular mass interferences by precipitation, and samples are re-tested after re-centrifugation (see Table 2) (33,34). However, sometimes the results are inconclusive. In general, the lower the recovery, the more likely is such an interference. If this test suggests interferences, the sample should be tested for the presence of heterophilic antibodies as well.
Table 2
| Step | Procedure |
|---|---|
| 1. | Mix 200 µL of plasma with 200 µL of 25% PEG 6000 (w/v) in phosphate buffered saline |
| 2. | Vortex this mixture for at least 20 seconds |
| 3. | Centrifugation for 10 minutes at ≥2,500 g |
| 4. | Re-test the supernatant |
| 5. | Calculate the recovery (%) as cTn [(post PEG) × 2/(cTn pre PEG)] × 100; a recovery of <35% suggests analytical interference |
Heterophilic antibodies can be unmasked by pre-incubation (usually for 10–15 minutes) of the sample in specific commercially available tubes (e.g., heterophilic blocking tube, Scandibodies Laboratory Inc., Santee, CA, USA) including so called heterophilic antibody blocking antibodies targeted against human anti-species antibodies or by pre-incubation of the sample with adding commercially available additional blocking antibodies (e.g., heterophilic blocking reagent, Scantibodies Laboratory Inc., Santee, CA, USA) before re-testing. These methods were used in several studies on cTn-assay interference (29-32). Blocking reagents eliminate interfering antibodies in many but not in all cases. A very significant change (about 95%) in test results is found, if heterophilic antibodies are eliminated by pre-treatment of the sample (29). Controls must be included with these commercially available kits to pre-treat samples to demonstrate that this procedure does not affect the immunoassay.
Rheumatoid factor
These are patient autoantibodies of the immunoglobulin M class directed against their own antibodies of the immunoglobulin G class usually binding to the Fc region (35,36). Low affinity crossreactivity with animal antibodies is frequent. They can be found in large quantities in autoimmune diseases, such as rheumatoid arthritis, lupus erythematodes or polymyositis. With current hs-cTn assay generation these antibodies rarely lead to false test results as blocking agents are added to the assay reagents. Therefore, interferences are only likely at very high titres of these antibodies. In these cases, PEG precipitation could be used to identify this antibody interference.
cTn autoantibodies
Circulating anti-cTnI and anti-cTnT autoantibodies have been reported in the literature (37-39). They usually cause assay dependent false negative test results by direct binding to the cTn epitope of the reagent antibody or in its vicinity, e.g., by blocking the binding of the test antibodies to the middle region of cTnI (38,39). At very high titers, they can mask the release of small amounts of cTnI, but their clinical significance is still uncertain. Autoantibodies against cTn can occur in association with autoimmune disorders, and an association between the presence of autoantibodies to cTnI and cardiomyopathy has been described as well (40).
Macro-troponins
Anti-cTn antibodies may also form so called macro-cTnI and macro-cTnT which may cause elevated hs-cTnI and hs-cTnT test results (37-44). An assay and patient population dependent wide range (0–55%) of their prevalence has been reported (37-42). In a systematic review of reported cases, they were the 3rd most frequent cause of false positive cTn test results (7). Macro-analytes consist of an analyte bound to analyte-specific autoantibodies (usually immunoglobulin G, e.g., anti-cTnI or anti-cTnT antibodies) resulting in high molecular mass complexes which may directly interfere with cTn assays leading to false-positive or false-negative test results (see Figures 2-4,6). However, macro-cTn complexes are eliminated more slowly from the circulation than the free analyte, resulting in persistently elevated cTn concentrations, that are not caused by analytical interference with the cTn assay.
Antibodies against the cTnI-cardiac troponin C complex or the whole cTn complex have been reported as well (41,42). Immunoglobulin-bound and unbound cTn may coexist in blood. Anti-cTn autoantibodies are more frequent in patients with a clinical history of myocardial injury (40,42) and may also develop as cross-reacting antibodies, e.g., after viral infections, during the course of autoimmune diseases or after treatment with checkpoint inhibitors. Their clinical significance is still debated (40,42). According to published data it appears that macro-cTn interference is more common in hs-cTnI assays than in the hs-cTnT assay (42).
Macro-cTn in a sample can be removed by PEG precipitation (34,42,43). This pre-treatment (see Table 2) non-specifically precipitates high molecular mass proteins including immunoglobulins. This kind of interference can be proven by more sophisticated, time-consuming techniques that separate macro-cTn complexes due to their higher molecular weight from cTn and its fragments, such as gel filtration chromatography [e.g., on a Sephadex G100 column (42-44)] or by western-blotting with an anti-cTn antibody, ideally using one of the cTn antibodies which are used in the cTn assay, and an anti-human immunoglobulin G antibody (45). In case of macro-cTn both antibodies stain the same lane, which has a markedly higher molecular mass than the respective cTn control lane (45). Alternatively, protein A/G pulldown could be also performed to prove this kind of analytical interference (34,42,43).
Unexpected cTn test result mismatches in specific patient populations
The simplest and quickest method to identify a false troponin test result usually is to re-test cTn in a newly drawn blood sample and/or sample type (if possible with the cTn assay in use) and to re-test cTn with an assay from another manufacturer (ideally measuring the alternative cardiac specific cTn isoform). A significantly different cTn result with an alternative cTn testing methodology in patients without clear clinical or imaging evidence for myocardial injury raises the suspicion of an analytical interference with one of these assays. However, this simple “trick” has limited sensitivity (42) and specificity for the detection of analytical interferences. Mismatches between cTnI and cTnT, i.e., increased cTnT but cTnI concentrations within the reference interval, may be found during the subacute phase of acute myocardial injury, because, e.g., after large AMIs cTnT tends to stay increased longer than cTnI (1). The typical clinical history of these patients, however, usually explains these cTn discrepancies. Additionally, there are specific patient populations without acute myocardial injury in whom during the work-up of unexpectedly increased cTn test results mismatches between cTnT and cTnI are relatively frequently found. These are patients with severe chronic renal failure and patients with chronic skeletal muscle diseases.
Chronic renal failure
Proteolytic cleavage in the bloodstream, capture of cTn by the reticuloendothelial system, metabolism in organs with high metabolic rate (e.g., liver) including the kidneys are probably involved in the elimination of cTn from the circulation. Interestingly, in stable patients with severe chronic renal failure without cardiac symptoms the percentage of increased hs-cTnT compared with hs-cTnI in these patients is markedly higher (46-48). The underlying causes are not fully understood yet and are probably multifactorial. They include chronic myocardial injury, at low cTn concentrations (<100 ng/L) impaired renal clearance is probably also involved (49,50). cTn fragments would be small enough for glomerular filtration, excretion in the renal tubular system could also contribute to cTn elimination. After hemodialysis, a significantly greater reduction of approximately 50% for hs-cTnT has been reported as compared to hs-cTnI (median 30%) in patients with end-stage renal failure (48). This discrepancy may be explained by potentially membrane specific differences of adherence of cTnI and cTnT and their immunoreactive complexes or fragments to the dialyser membrane. Patients with end-stage renal failure may be also affected by uremic skeletal muscle myopathy. Reports on cTnT expression in skeletal muscles of patients with end-stage renal failure are still conflicting (51,52). Haller et al. published the lack of cTnT expression in abdominal wall or back skeletal muscle biopsy specimen of 5 patients with end-stage renal failure (52). However, it needs to be considered, that truncal skeletal muscles are typically not involved in uremic skeletal myopathy, it usually affects proximal-extremity muscles. By contrast, Ricchiuti and Apple (51) reported cTnT mRNA expression and cTnT protein expression detected by Western blotting without evidence for cTnI expression in about 50% of skeletal muscle specimens of hemodialysis patients. In these patients, abdominal wall, back muscles and arm muscles were tested. On the other hand, Zümrütdal et al. (53) found only a weak relationship between the presence of uremic skeletal muscle myopathy and elevated cTnT concentrations in 50 chronic hemodialysis patients. Despite more frequently seen cTnT increases and irrespective of the mechanisms of cTn increase, both hs-cTnT and hs-cTnI maintain their prognostic value in patients with chronic renal failure (48,54-57). However, in the setting of severe chronic renal failure patients with suspected AMI, the clinical specificity of hs-cTnI appears to be superior to hs-cTnT (58,59). It is important to stress, that it is always mandatory to detect a significant cTn concentration change in serial testing for the definitive diagnosis of acute myocardial injury (1-6).
Patients with chronic skeletal muscle diseases
During fetal development, cTnT is expressed in cardiac and skeletal muscle. In skeletal muscle, cTnT is downregulated during development, cTnT gradually disappears after birth. Healthy human adult skeletal muscle, therefore, does not express cTnT anymore (60-66). By contrast, cTnI is not expressed in fetal and adult human skeletal muscle (54). In contrast to cTnI, embryonic and adult cTnT splice variants have been also reported (cTnT isoforms), which are formed after transcription of the gen by alternative processing of the mRNA before translation into proteins (60), which leads to a greater diversity of isoforms.
When human skeletal muscle is chronically damaged, such as in patients with chronic skeletal muscle myopathy or chronic myositis, it re-expresses fetal proteins, possibly including cTnT isoforms. Such a process is likely dependent on the severity and duration of the disease. These re-expressed fetal proteins may be released into the bloodstream from chronically damaged skeletal muscle. Previous studies in human skeletal muscle biopsies detected cTnT coding messenger ribonucleid acid (mRNA) and re-expression of cTnT in skeletal muscle at the protein level by immunohistochemistry and/or western-blotting (60-72). Given the inherent problems of specificity of the latter methods, these reports cannot be taken as definitive proof of cTnT protein re-expression. Recently, however, cTnT fragments could be detected by using nanoflow liquid chromatography/mass spectrometry (LC-MS) mass spectrometry in skeletal muscle biopsy specimens of some patients with Pompe disease (73). This could be confirmed by simultaneous detection of mRNA (73). Wens et al. (73) detected peptide fragments of the cTnT isoform 6, which is the cTnT isoform expressed in healthy hearts. cTnT was not detected in skeletal muscle of healthy controls (67,73). Thus, in patients with Pompe disease skeletal muscle is a potential source of increased cTnT concentrations. By contrast, Schmid et al. (24) reported no evidence for cTnT re-expression in skeletal muscle biopsies of patients with myopathies and myositis by mass-spectrometry, despite markedly more patients showing increased cTnT concentrations in peripheral blood compared with cTnI despite lack of evidence for cTnT re-expression. Finally, despite accumulating evidence of cTnT expression in several chronic skeletal muscle diseases the available data is still inconclusive and this issue is currently not definitively solved. Clinically, however, the most cardiac-specific marker in this rare population of patients with chronic skeletal muscle damage (e.g., muscular dystrophy or chronic myositis) appears to be cTnI, which is easier to interpret when AMI is suspected in these patients.
Summary
It is important to stress that the vast majority of unexpectedly, increased cTn concentrations in daily clinical practice is caused by acute or chronic myocardial injury (the possible underlying diseases are summarized in Table 1), which may not be obvious at first sight but is usually plausible when aggregating all available clinical information. Myocardial injury is much more common in various clinical settings than previously known, and, e.g., Lindner et al. (74) reported in a cross-sectional analysis of emergency department samples with increased cTn values without overt myocardial injury that renal failure and acute cerebral events were the most common non-AMI-related causes of elevated hs-cTnT. Usually in these patients subsequent imaging for ruling out significant structural or coronary heart disease is additionally needed (see Figure 1). However, for the detection of myocardial injury, hs-cTn testing appears to be more sensitive than all currently available imaging modalities including cardiac magnetic resonance imaging (75). In general, perhaps apart from athletes after heavy bouts of extraordinary endurance exercise (76), increased cTnI and cTnT concentrations should be considered as evidence for myocardial injury indicating adverse prognosis (77).
However, in cases where cTn test results and the clinical picture are strikingly different, outliers and analytical false positive or false-negative test results should also be considered. In such non-critically ill patients, the laboratory should be contacted first before a costly or invasive work-up is started, particularly in patient populations with a theoretically higher risk for antibody interferences (e.g., autoimmune diseases, after myocarditis or treatment with monoclonal antibodies). As a first step after re-testing the sample after re-centrifugation, an additional blood sample should be taken to exclude the following simple reasons, i.e., random error or mix-up of samples or fibrin micro clots or other microparticles in the specimen. If available, the second and first sample should be measured with another hs-cTn assay, ideally testing the other cardiac-specific cTn isoform. This gives the quickest answer in daily clinical practice. It must be stressed, that linearity in serial dilution, another simple screening procedure, does not reliably rule out analytical interferences, in particular macro-cTn (45). A persistent cTn test result that does not fit the clinical picture, especially when combined with a consistent alternative cTn test result, raises suspicion of an analytical interference. In such situations clinicians should follow the cTn test result that fits to the clinical presentation for initial patient care.
As outlined above, subsequently more time consuming but still simple procedures, e.g., precipitation with PEG 6000 or pre-incubation with heterophilic antibody blocking reagents before re-testing can be easily performed to rule out the most common interferences in most hospital laboratories. If, e.g., dilution screening testing suggests an interference, these subsequent confirmatory tests are mandatory to prove an analytical interference, because of the limitations of this screening test. If these confirmatory tests cannot demonstrate an analytical interference, an analytical cause is unlikely. However, all these simple methods have limited sensitivities for the detection of analytical interferences. The identification or exploring the exact nature of a potentially interfering substance may require more sophisticated and time-consuming analytical procedures, necessitating collaboration with the test manufacturer or a specialized laboratory.
To have a cTn back-up assay in case of questionable cTn test results is a good option for tertiary care centres to quickly check for analytical interferences with a cTn assay. It should be noted, however, that the sensitivity and specificity of this simple “trick” are limited. In patients with chronic skeletal muscle disease or severe chronic renal failure without cardiac symptoms, elevated cTnT concentrations are more common than elevated cTnI concentrations. The exact mechanisms leading to this phenomenon are still under debate and are not definitively known yet. From a clinical perspective, in case of suspected AMI in these patient populations the specificity of cTnI is higher.
Strengths and limitations of this review
This review has been written by two experienced experts in the field covering laboratory as well as clinical aspects of the work-up of questionable troponin test results based on the published literature on this topic, which, however, mainly comprises only of reports of cases or case series. Analytical interferences and outliers are assay dependent. Therefore, it is impossible to report a generally valid prevalence of this problem in daily clinical practice.
Conclusions
Analytical interferences are rare but are not harmless, because they may lead to misdiagnosis, mismanagement of patients, and excess diagnostic testing. An unexpectedly, increased cTn test result should be regarded as a sign of myocardial injury until an outlier or an analytical interference has been proven or could be identified as a very likely cause. In the vast majority of these patients, cTn test results are due to myocardial injury. However, a significantly elevated cTn in an otherwise healthy individual with a very low clinical probability of cardiac disease should raise suspicion of a false-positive test result, which should be excluded, time permitting, before initiating extensive and costly or invasive clinical investigations. For this purpose, close collaborative efforts between clinicians and laboratorians are needed. Outliers appear to be the most common cause of false positive cTn test results in daily clinical practice. They should be ruled-out before time consuming testing for rare analytical interferences which are most frequently caused by heterophilic antibodies and macro-cTn.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Xander van Wijk, Amy Saenger, Steven Meex, and Allan Jaffe) for the series “Cardiac Troponin” published in Journal of Laboratory and Precision Medicine. The article has undergone external peer review.
Peer Review File: Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-22-65/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-22-65/coif). The series “Cardiac Troponin” was commissioned by the editorial office without any funding or sponsorship. JM reports research collaboration on cardiac biomarker point of care diagnostics with Siemens Healthineers, the Netherlands. OH has received the support and grants from the Swedish Cancer Society, the Swedish Heart and Lung Foundation and LUA/ALF funding at the Sahlgrenska University Hospital, and received consult fee from LumiraDx, payments from Siemens Healthineers and stock from Aligned Bio. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thygesen K, Mair J, Katus H, et al. Recommendations for the use of cardiac troponin measurement in acute cardiac care. Eur Heart J 2010;31:2197-204. [Crossref] [PubMed]
- Thygesen K, Mair J, Giannitsis E, et al. How to use high-sensitivity cardiac troponins in acute cardiac care. Eur Heart J 2012;33:2252-7. [Crossref] [PubMed]
- Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J 2019;40:237-69. [Crossref] [PubMed]
- Mair J, Cullen L, Giannitsis E, et al. Application of the fourth universal definition of myocardial infarction in clinical practice. Biomarkers 2020;25:322-30. [Crossref] [PubMed]
- Lindahl B, Baron T, Albertucci M, et al. Myocardial infarction with non-obstructive coronary artery disease. EuroIntervention 2021;17:e875-87. [Crossref] [PubMed]
- Mair J, Lindahl B, Müller C, et al. What to do when you question cardiac troponin values. Eur Heart J Acute Cardiovasc Care 2018;7:577-86. [Crossref] [PubMed]
- Nevraumont A, Deltombe M, Favresse J, et al. Interferences with cardiac biomarker assays: understanding the clinical impact. Eur Heart J 2022;43:2286-8. [Crossref] [PubMed]
- Apple FS, Collinson PO. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem 2012;58:54-61. [Crossref] [PubMed]
- Yeo KT, Storm CA, Li Y, et al. Performance of the enhanced Abbott AxSYM cardiac troponin I reagent in patients with heterophilic antibodies. Clin Chim Acta 2000;292:13-23. [Crossref] [PubMed]
- Krintus M, Kozinski M, Boudry P, et al. European multicenter analytical evaluation of the Abbott ARCHITECT STAT high sensitive troponin I immunoassay. Clin Chem Lab Med 2014;52:1657-65. [Crossref] [PubMed]
- Fitzgerald RL, Hollander JE, Peacock WF, et al. Analytical performance evaluation of the Elecsys® Troponin T Gen 5 STAT assay. Clin Chim Acta 2019;495:522-8. [Crossref] [PubMed]
- Krebber A, Bornhauser S, Burmester J, et al. Reliable cloning of functional antibody variable domains from hybridomas and spleen cell repertoires employing a reengineered phage display system. J Immunol Methods 1997;201:35-55. [Crossref] [PubMed]
- Lum G, Solarz DE, Farney L. False positive cardiac troponin results in patients without myocardial infarction. Lab Med 2006;37:546-50. [Crossref]
- Vafaie M, Biener M, Mueller M, et al. Analytically false or true positive elevations of high sensitivity cardiac troponin: a systematic approach. Heart 2014;100:508-14. [Crossref] [PubMed]
- Pretorius CJ, Dimeski G, O'Rourke PK, et al. Outliers as a cause of false cardiac troponin results: investigating the robustness of 4 contemporary assays. Clin Chem 2011;57:710-8. [Crossref] [PubMed]
- Karon BS, Wockenfus AM, Hartung KJ, et al. Comparing analytical outliers and the percent of emergency department patients with results above the 99th percentile upper reference limit for 2 conventional and one high sensitivity troponin assay. Clin Biochem 2018;53:104-9. [Crossref] [PubMed]
- Sawyer N, Blennerhassett J, Lambert R, et al. Outliers affecting cardiac troponin I measurement: comparison of a new high sensitivity assay with a contemporary assay on the Abbott ARCHITECT analyser. Ann Clin Biochem 2014;51:476-84. [Crossref] [PubMed]
- Harley K, Bissonnette S, Inzitari R, et al. Independent and combined effects of biotin and hemolysis on high-sensitivity cardiac troponin assays. Clin Chem Lab Med 2021;59:1431-43. [Crossref] [PubMed]
- Trambas C, Lu Z, Yen T, et al. Characterization of the scope and magnitude of biotin interference in susceptible Roche Elecsys competitive and sandwich immunoassays. Ann Clin Biochem 2018;55:205-15. [Crossref] [PubMed]
- Dasgupta A, Khalil S. Effect of Biotin on Cardiac Troponin I and High Sensitivity Cardiac Troponin I Assays on Vista 1500 and ADVIA Centaur Analyzer. Ann Clin Lab Sci 2021;51:102-5. [PubMed]
- Mumma B, Diercks D, Twerenbold R, et al. Clinical risk assessment of biotin interference with a high-sensitivity cardiac troponin T assay. Clin Chem Lab Med 2020;58:1931-40. [Crossref] [PubMed]
- Collinson P. Biotin interference in cardiac troponin immunoassay - where the wild things are? Clin Chem Lab Med 2020;58:1769-71. [Crossref] [PubMed]
- Herman DS, Ranjitkar P, Yamaguchi D, et al. Endogenous alkaline phosphatase interference in cardiac troponin I and other sensitive chemiluminescence immunoassays that use alkaline phosphatase activity for signal amplification. Clin Biochem 2016;49:1118-21. [Crossref] [PubMed]
- Schmid J, Liesinger L, Birner-Gruenberger R, et al. Elevated Cardiac Troponin T in Patients With Skeletal Myopathies. J Am Coll Cardiol 2018;71:1540-9. [Crossref] [PubMed]
- Rulander NJ, Cardamone D, Senior M, et al. Interference from anti-streptavidin antibody. Arch Pathol Lab Med 2013;137:1141-6. [Crossref] [PubMed]
- Gessl A, Blueml S, Bieglmayer C, et al. Anti-ruthenium antibodies mimic macro-TSH in electrochemiluminescent immunoassay. Clin Chem Lab Med 2014;52:1589-94. [Crossref] [PubMed]
- Bolstad N, Warren DJ, Nustad K. Heterophilic antibody interference in immunometric assays. Best Pract Res Clin Endocrinol Metab 2013;27:647-61. [Crossref] [PubMed]
- Klee GG. Human anti-mouse antibodies. Arch Pathol Lab Med 2000;124:921-3. [Crossref] [PubMed]
- Lippi G, Aloe R, Meschi T, et al. Interference from heterophilic antibodies in troponin testing. Case report and systematic review of the literature. Clin Chim Acta 2013;426:79-84. [Crossref] [PubMed]
- Franeková J, Bláha M, Bělohoubek J, et al. A clinical and laboratory approach used to elucidate discordant results of high-sensitivity troponin T and troponin I. Clin Chim Acta 2015;446:128-31. [Crossref] [PubMed]
- Baroni S, Troiani E, Santonocito C, et al. A false positive case of high-sensitivity cardiac troponin in a patient with acute chest pain: Analytical study of the interference. Clin Biochem 2019;66:103-5. [Crossref] [PubMed]
- Hedley J, Menon V, Cho L, et al. Fifth generation troponin T assay is subject to antibody interference. Clin Chim Acta 2020;505:98-9. [Crossref] [PubMed]
- Lam L, Ha L, Gladding P, et al. Effect of macrotroponin on the utility of cardiac troponin I as a prognostic biomarker for long term total and cardiovascular disease mortality. Pathology 2021;53:860-6. [Crossref] [PubMed]
- Lafrenière MA, Tandon V, Ainsworth C, et al. Storage conditions, sample integrity, interferences, and a decision tool for investigating unusual high-sensitivity cardiac troponin results. Clin Biochem 2022;S0009-9120(22)00147-3.
- Kenny PR, Finger DR. Falsely elevated cardiac troponin-I in patients with seropositive rheumatoid arthritis. J Rheumatol 2005;32:1258-61. [PubMed]
- Marinheiro R, Amador P, Parreira L, et al. False Positive Troponin I Rendering Two Admissions for "Recurrent Acute Myopericarditis". Open Cardiovasc Med J 2018;12:55-8. [Crossref] [PubMed]
- Eriksson S, Hellman J, Pettersson K. Autoantibodies against cardiac troponins. N Engl J Med 2005;352:98-100. [Crossref] [PubMed]
- Adamczyk M, Brashear RJ, Mattingly PG. Prevalence of autoantibodies to cardiac troponin T in healthy blood donors. Clin Chem 2009;55:1592-3. [Crossref] [PubMed]
- Savukoski T, Jacobino J, Laitinen P, et al. Novel sensitive cardiac troponin I immunoassay free from troponin I-specific autoantibody interference. Clin Chem Lab Med 2014;52:1041-8. [Crossref] [PubMed]
- Shmilovich H, Danon A, Binah O, et al. Autoantibodies to cardiac troponin I in patients with idiopathic dilated and ischemic cardiomyopathy. Int J Cardiol 2007;117:198-203. [Crossref] [PubMed]
- Warner JV, Marshall GA. High incidence of macrotroponin I with a high-sensitivity troponin I assay. Clin Chem Lab Med 2016;54:1821-9. [Crossref] [PubMed]
- Lam L, Aspin L, Heron RC, et al. Discrepancy between Cardiac Troponin Assays Due to Endogenous Antibodies. Clin Chem 2020;66:445-54. [Crossref] [PubMed]
- Lam L, Ha L, Heron C, et al. Identification of macrotroponin T: findings from a case report and non-reproducible troponin T results. Clin Chem Lab Med 2021;59:1972-80. [Crossref] [PubMed]
- Broz P, Racek J, Prokop P, et al. Macrotroponins cause discrepancy in high-sensitivity examination. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2023; Epub ahead of print. [Crossref] [PubMed]
- Akhtar Z, Dargan J, Gaze D, et al. False-positive troponin elevation due to an immunoglobulin-G-cardiac troponin T complex: a case report. Eur Heart J Case Rep 2020;4:1-5. [Crossref] [PubMed]
- Chung JZ, Dallas Jones GR. Effect of renal function on serum cardiac troponin T--Population and individual effects. Clin Biochem 2015;48:807-10. [Crossref] [PubMed]
- Badiou S, Boudet A, Leray-Moragues H, et al. Monthly reference change value of cardiac troponin in hemodialysis patients as a useful tool for long-term cardiovascular management. Clin Biochem 2016;49:1195-8. [Crossref] [PubMed]
- Buiten MS, de Bie MK, Rotmans JI, et al. Serum Cardiac Troponin-I is Superior to Troponin-T as a Marker for Left Ventricular Dysfunction in Clinically Stable Patients with End-Stage Renal Disease. PLoS One 2015;10:e0134245. [Crossref] [PubMed]
- Fridén V, Starnberg K, Muslimovic A, et al. Clearance of cardiac troponin T with and without kidney function. Clin Biochem 2017;50:468-74. [Crossref] [PubMed]
- Muslimovic A, Fridén V, Tenstad O, et al. The Liver and Kidneys mediate clearance of cardiac troponin in the rat. Sci Rep 2020;10:6791. [Crossref] [PubMed]
- Ricchiuti V, Apple FS. RNA expression of cardiac troponin T isoforms in diseased human skeletal muscle. Clin Chem 1999;45:2129-35. [Crossref] [PubMed]
- Haller C, Zehelein J, Remppis A, et al. Cardiac troponin T in patients with end-stage renal disease: absence of expression in truncal skeletal muscle. Clin Chem 1998;44:930-8. [Crossref] [PubMed]
- Zümrütdal A, Bakinen O, Uçan H, et al. Relationship between uremic myopathy and false-positive cardiac troponin T test. Nephron 2000;86:522-3. [Crossref] [PubMed]
- McGill D, Talaulikar G, Potter JM, et al. Over time, high-sensitivity TnT replaces NT-proBNP as the most powerful predictor of death in patients with dialysis-dependent chronic renal failure. Clin Chim Acta 2010;411:936-9. [Crossref] [PubMed]
- Hickman PE, McGill D, Potter JM, et al. Multiple biomarkers including cardiac troponins T and I measured by high-sensitivity assays, as predictors of long-term mortality in patients with chronic renal failure who underwent dialysis. Am J Cardiol 2015;115:1601-6. [Crossref] [PubMed]
- deFilippi C, Seliger SL, Kelley W, et al. Interpreting cardiac troponin results from high-sensitivity assays in chronic kidney disease without acute coronary syndrome. Clin Chem 2012;58:1342-51. [Crossref] [PubMed]
- Sandoval Y, Herzog CA, Love SA, et al. Prognostic Value of Serial Changes in High-Sensitivity Cardiac Troponin I and T over 3 Months Using Reference Change Values in Hemodialysis Patients. Clin Chem 2016;62:631-8. [Crossref] [PubMed]
- Twerenbold R, Wildi K, Jaeger C, et al. Optimal Cutoff Levels of More Sensitive Cardiac Troponin Assays for the Early Diagnosis of Myocardial Infarction in Patients With Renal Dysfunction. Circulation 2015;131:2041-50. [Crossref] [PubMed]
- Huang H, Zhu S, Wang W, et al. Diagnosis of acute myocardial infarction in patients with renal insufficiency using high-sensitivity troponin T. Clin Chem Lab Med 2015;53:723-30. [Crossref] [PubMed]
- Rasmussen M, Jin JP. Troponin Variants as Markers of Skeletal Muscle Health and Diseases. Front Physiol 2021;12:747214. [Crossref] [PubMed]
- Wei B, Jin JP. TNNT1, TNNT2, and TNNT3: Isoform genes, regulation, and structure-function relationships. Gene 2016;582:1-13. [Crossref] [PubMed]
- Wei B, Jin JP. Troponin T isoforms and posttranscriptional modifications: Evolution, regulation and function. Arch Biochem Biophys 2011;505:144-54. [Crossref] [PubMed]
- Saggin L, Gorza L, Ausoni S, et al. Cardiac troponin T in developing, regenerating and denervated rat skeletal muscle. Development 1990;110:547-54. [Crossref] [PubMed]
- Anderson PA, Malouf NN, Oakeley AE, et al. Troponin T isoform expression in humans. A comparison among normal and failing adult heart, fetal heart, and adult and fetal skeletal muscle. Circ Res 1991;69:1226-33. [Crossref] [PubMed]
- Sabry MA, Dhoot GK. Identification of and pattern of transitions of cardiac, adult slow and slow skeletal muscle-like embryonic isoforms of troponin T in developing rat and human skeletal muscles. J Muscle Res Cell Motil 1991;12:262-70. [Crossref] [PubMed]
- Mesnard L, Samson F, Espinasse I, et al. Molecular cloning and developmental expression of human cardiac troponin T. FEBS Lett 1993;328:139-44. [Crossref] [PubMed]
- du Fay de Lavallaz J, Prepoudis A, et al. Skeletal Muscle Disorders: A Noncardiac Source of Cardiac Troponin T. Circulation 2022;145:1764-79. [Crossref] [PubMed]
- Bodor GS, Survant L, Voss EM, et al. Cardiac troponin T composition in normal and regenerating human skeletal muscle. Clin Chem 1997;43:476-84. [Crossref] [PubMed]
- Messner B, Baum H, Fischer P, et al. Expression of messenger RNA of the cardiac isoforms of troponin T and I in myopathic skeletal muscle. Am J Clin Pathol 2000;114:544-9. [Crossref] [PubMed]
- Hammerer-Lercher A, Erlacher P, Bittner R, et al. Clinical and experimental results on cardiac troponin expression in Duchenne muscular dystrophy. Clin Chem 2001;47:451-8. [Crossref] [PubMed]
- McLaurin MD, Apple FS, Voss EM, et al. Cardiac troponin I, cardiac troponin T, and creatine kinase MB in dialysis patients without ischemic heart disease: evidence of cardiac troponin T expression in skeletal muscle. Clin Chem 1997;43:976-82. [Crossref] [PubMed]
- Jaffe AS, Vasile VC, Milone M, et al. Diseased skeletal muscle: a noncardiac source of increased circulating concentrations of cardiac troponin T. J Am Coll Cardiol 2011;58:1819-24. [Crossref] [PubMed]
- Wens SC, Schaaf GJ, Michels M, et al. Elevated Plasma Cardiac Troponin T Levels Caused by Skeletal Muscle Damage in Pompe Disease. Circ Cardiovasc Genet 2016;9:6-13. [Crossref] [PubMed]
- Lindner G, Pfortmueller CA, Braun CT, et al. Non-acute myocardial infarction-related causes of elevated high-sensitive troponin T in the emergency room: a cross-sectional analysis. Intern Emerg Med 2014;9:335-9. [Crossref] [PubMed]
- Selvanayagam JB, Porto I, Channon K, et al. Troponin elevation after percutaneous coronary intervention directly represents the extent of irreversible myocardial injury: insights from cardiovascular magnetic resonance imaging. Circulation 2005;111:1027-32. [Crossref] [PubMed]
- Möhlenkamp S, Leineweber K, Lehmann N, et al. Coronary atherosclerosis burden, but not transient troponin elevation, predicts long-term outcome in recreational marathon runners. Basic Res Cardiol 2014;109:391. [Crossref] [PubMed]
- Roos A, Bandstein N, Lundbäck M, et al. Stable High-Sensitivity Cardiac Troponin T Levels and Outcomes in Patients With Chest Pain. J Am Coll Cardiol 2017;70:2226-36. [Crossref] [PubMed]
Cite this article as: Mair J, Hammarsten O. Potential analytical interferences in cardiac troponin immunoassays. J Lab Precis Med 2023;8:12.


