
A practical guide to the implementation of high-sensitivity cardiac troponin assays into the clinical lab
Introduction
Throughout its history with advancements in medical technology, the clinical laboratory has undergone improvements of analytical methods used for diagnostic purposes, particularly for analytical sensitivity and precision. This has resulted in better differentiation between health and disease states. For example, assays for thyroid stimulating hormone (TSH) have undergone various generations defined by analytical sensitivity. Starting with the first generation TSH assay which had a functional sensitivity of 1 mIU/mL (defined as the value with a 20% imprecision), each successive assays have lowered that sensitivity by 10-fold per generation which have improved the diagnostic utility of this test (1). While the original C-reactive protein (C-RP) test was useful for detecting systemic inflammation, high-sensitivity C-RP is useful for detecting sub-clinical inflammation and is a risk factor for cardiovascular disease (2).
Cardiac troponin (cTn) has also undergone improvements in analytical sensitivity that have led to superior performance for acute myocardial infarction (AMI) diagnosis and created new medical indications for the test that was not previously possible with other cardiac biomarkers or the first generation cTn test. The first-generation cTn test was used to diagnose AMI and therefore had similar performance to the existing standard tests of creatine kinase (CK)-muscle and cardiac (MB) isoenzyme. The definition of a high sensitivity (hs) assay was coined by Apple, who suggested that the assay must be able to report values above the assays limit of detection for more than 50% of all healthy subjects tested (3). With the implementation of hs-troponin, earlier rule out of AMI can be performed (4). Figure 1 illustrates the evolution of cutoff concentrations for various generations of cTn assays.
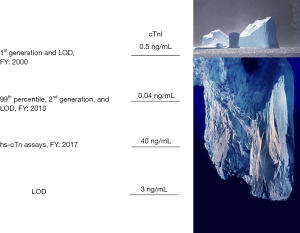
Implementation of any new assay or generation of an existing assay requires careful consideration by various stakeholders. In 2019, an expert panel of the American College of Cardiology provided recommendations for hospitals wishing to adopt high-sensitive troponin assays (5). At that time, Food and Drug Administration (FDA) approval for high-sensitivity cTnT and cTnI assays had just emerged. Implementation of these assays were slowed, with the focus having been diverted towards managing the COVID-19 pandemic. Now that the pandemic has been more manageable, the focus can be re-directed towards implementing hs-troponin assays. At some point, manufacturers of reagents will discontinue production of previous generation assays. Therefore, it will be essential for hospitals who have not adopted these assays to begin the transition process as soon as possible.
Identify and collaborate with stake holders
Implementation of hs-troponin assays has impact in several areas within the hospital, but most notably the clinical laboratory, emergency department (ED), the division of cardiology and information technology. The successful launch of hs-troponin testing will require planning with representative members of each group providing input. The implementation plan can be driven by any of these groups. Once this team is assembled, the agenda include understanding the reason for change, the timeline for troponin assay validation, modification of blood collection frequency, establishing cutoff concentrations and delta change of serial results for each time point, establishing a care pathway through the electronic medical records system, and the education of users (doctors, physician assistants, nurses, and lab personnel). There should also be a post-implementation plan to evaluate the acceptance by physicians, and success of the pathway using acceptable metrics.
Alteration of blood collection frequency for testing patients presenting with chest pain
To take full advantage of hs-troponin, the time interval of blood collections, relative to the first collection at the time of the patient’s presentation to the ED, must be reduced. Using conventional biomarker such as CK-MB and myoglobin, and first-generation troponin assays, the typical pattern for blood collection at most EDs was either admission, 4, and 8 hours, or admission, 3, 6 and even 9 hours. The major advantage of hs-cTn assays is early rule out of AMI from patients who present to the ED with chest pain. Figure 2 shows how the use of hs-troponin enables early rule in and rule out of AMI. A faster rule out facilitates a decision for faster discharge. With hs-cTn values that are at or near the limit of detection, rule out of AMI can be accomplished with a single troponin result, if the onset of chest pain is documented to be at least 3 hours (6). For both rule-in and rule-out, the accelerated testing protocol saves ED resources (both personnel and bedspace).
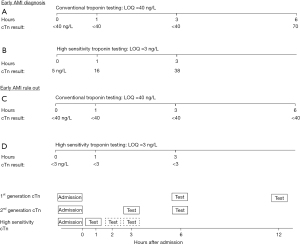
Implementation of an accelerated of an accelerated diagnostic pathway requires that laboratory to consistently report testing results within one hour from the time of specimen collection (not the test order). This is a challenge today for many central laboratories. Ideally, results of the first cTn test should be available before the collection of the second sample.
The accelerated diagnostic protocol can still be implemented even if the result of the first sample is not completed when the second sample needs to be collected. Cutoff concentrations for serial testing are specifically linked to the time interval between collections. Therefore, it is important to adhere to the schedule of draws as inaccurate interpretations could occur if the timing is not aligned. Reductions in turnaround times (TAT) can be achieved by understanding the sources of delays and implementing a multidisciplinary process improvement plan (7). In particular, the ED nursing and specimen delivery staff must be cooperative. Use of point-of-care testing (POCT) devices could also facilitate a more rapid TAT (see below).
Adjustment of quality control materials target range and change in units of measure
cTn is one of the clinical chemistry tests where high analytical sensitivity is required to maximize the full benefit of this biomarker. Among the other examples include measurement of TSH for hyperthyroidism diagnosis, tumor markers such as prostate specific antigen to determine minimal residual disease detection, and high-sensitivity C-RP as a cardiovascular disease risk assessment marker. Verification of daily test results is achieved with testing of quality control materials. The value assignments of these materials must challenge the limits of analytical sensitivity. Adoption of controls for conventional troponin assays are insufficient as they are not low enough in target concentrations to challenge the lower sensitivity ranges. The Academy of the American Association for Clinical Chemistry has recommended three materials at the following ranges: (I) between the limit of detection and the lowest sex-specific 99th percentile, (II) within 20% of the highest sex-specific 99th percentile, and (III) a value that challenges the upper analytical range of reportable results (8).
The Academy and other groups (3) have recommended changing the units of measure from ng/mL to ng/L to comply with the International Units of Measure (SI) and to provide troponin values in whole numbers instead of fractions. While the procedure for physically changing units within a laboratory report is rather straight forward, notification of all physicians and caregivers must be carefully planned. As a further example of the improvement in interpretation, suppose the admission sample for a patient with chest pain had a conventional troponin value of 0.004 ng/mL, which increases to 0.039 ng/mL within 1 h (99th percentile cutoff 0.040 ng/mL). Is this an evolving AMI? Although not immediately obvious, this is a 10-fold increase which suggest an evolving AMI. Using units of ng/L, this would be a change from 4 to 39 (99th percentile cutoff 40 ng/L). While the second result has not exceeded the cutoff, the rapid rise of troponin makes and AMI diagnosis more evident.
Misinterpretation of a troponin result can result in a significant medical error. A value that is 1,000 times higher, e.g., from 0.040 ng/mL to 40 ng/L could be misconstrued as being strongly positive if the change in reporting unit is not recognized or appreciated by the medical team. Unnecessary treatment, such as a cardiac catheterization, which carries some medical risks, could occur if a 40 ng/L result is interpreted as grossly abnormal. The clinical laboratory must alert the medical staff with communications prior to implementation. On the day of implementation, direct communication with the ED staff as to the unit could be helpful. The electronic medical records system could provide notices of the change of units.
Along with these changes, the medical practice needs to decide if sex-specific reference intervals are to be adopted. Prior to the adoption of hs-cTn assays, sex-specific reference intervals were not possible because values the majority of healthy subjects did not have values within the measurement range. Not all clinicians, however, agree that sex-specific reference intervals are advantageous (9).
Education as to the interpretation of abnormal troponin
One of the biggest challenges in implementing hs-troponin is the overcoming the notion that troponin is a specific marker of AMI. Cutoff concentrations for CK (MB) isoenzyme were established to differentiate unstable angina from AMI, thus it is appropriate to consider this as an AMI biomarker (10). However, when cuttoff is established at the 99th percentile of healthy subjects, more abnormal cTn results are produced due to the presence of myocardial injury. While it is true that AMI is a common cause of heart damage, it is only one of many etiologies that can release troponin. Implementing hs assays will result in more medical conditions that are associated with cardiac damage. Therefore, the major educational hurdle is to rely the concept that troponin is a marker of cardiac damage and not just AMI. The in vitro diagnostics industry complicates this issue because troponin is approved through the FDA as a marker of AMI. Linking test results of conditions that are associated with cardiac injury is more difficult to adjudicate as a medical claim. Correlating test results to other FDA-approved cTn assays is an easier route to achieve clearance, but unfortunately perpetuates the misunderstanding of what this test indicates.
Education of the medical staff is a multi-step process regarding the implementation of high-sensitivity troponin assays. After identification of a local expert or key opinion leader within an institution, a series of educational seminars are warranted. Presentation to the medical staff should take place just before the implementation of the test. Guest speakers in the form of grand rounds may be invited to provide more national credence. While it is possible to obtain corporate support for speaker travel and honoria to get the largest audience, the session should offer continuing medical education credits. There sponsor must have no role in the content, and the lecture itself must not have any based in favor of the lecture sponsor. When the time draws nearer to implementation, more lectures are warranted directed at the nursing and laboratory staff. The focus of these talks should be on logistics and practical issues.
POCT
The interest in testing cTn within the ED has been discussed for many years. There have been many qualitative and quantitative whole-blood point-of-care devices approved by the FDA for use in the ED. These initial tests made use of lateral flow immunochromatography strip which was read on a handheld or small desk top reader. The TAT for reporting results is faster than sending samples to a central lab, and therefore it facilitates accelerated diagnostic pathway protocols. Unfortunately, none of these early devices qualified as a high-sensitivity assay. Next-generation POCT devices are on the horizon and when approved, they could replace some cTn testing from the central lab (11,12). The standardization of results obtained from POCT versus central testing remains is an ongoing problem. The costs for implementing POCT within the ED will also be higher and must be justified by faster rule out and patient flow. Despite the clear economic advantage of reducing ED length of stay, hospital budgets are individualized, making it more difficult for justify increasing costs for one department in order for cost savings from another. Strong hospital leadership can help departments see the bigger financial picture.
Post-hoc analysis
Once the implementation has taken place, it would be useful to perform a post-hoc quality improvement analysis. Such a study would promote good relations between the various major departments (cardiology, emergency medicine, clinical laboratory, and information technology). Among the metric that could be considered include adherence to the altered blood protocols, TAT for reporting cTn lab results, and time to initiate medical decision. Some of this information may be difficult to obtain from the medical records. As a comparative group, results for these indices could be obtained during a period before implementation of the troponin protocol. As some of this data could be published, the implementation team may need to consult local institutional review board for approval. A particularly powerful indicator would be to determine if the medial ED length of stay was decreased with the implementation of an accelerated AMI-rule out protocol. Unfortunately, ED discharge decisions are rarely dependent on the TAT for clinical lab results. Even if results for troponin were made available, there may be other tests that are pending. When a discharge decision is made by the medical team, there are other factors that dictate when the patient actually leaves. These factors include the time of day, availability of transportation, housing (in the case of homeless patients), address of the patient, availability of outpatient care, etc.
Future troponin assays
The current commercial high-sensitivity troponin assays can detect 50% to 80% of healthy subjects [International Federation of Clinical Chemistry and Laboratory Medicine (IFCC)] (10). There are a few experimental research assays that are not approved by the FDA that offer percentages approaching 100%. It is unclear at this stage if higher analytical sensitivity produces and additional advantages to the current generation of assays. In a similar manner, 4th generation TSH assays have been available for a few years. The advantages of this TSH assay is for detection of a greater faction of hyperthyroid patients relative to the 3rd generation assay, and monitoring their treatment (13). However, most commercial TSH assays still utilize the third generation. Research is ongoing to determine the pathophysiology of troponin release into blood and its subsequent degradation. For example, the measurement of C-terminal and N-terminal fragments of cTn may provide additional medical value (14). Assays with higher analytical sensitivity may needed to measure these lower molecular weight peptides. Once identified, novel clinical trials must be conducted to determine if there are diagnostic value for measuring the subfractions.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Xander van Wijk, Amy Saenger, Steven Meex, and Allan Jaffe) for the series “Cardiac Troponin” published in Journal of Laboratory and Precision Medicine. The article has undergone external peer review.
Peer Review File: Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-22-61/prf
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-22-61/coif). The series “Cardiac Troponin” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Demers LM, Spencer CA. National Academy of Clinical Biochemistry, Laboratory Medicine Practice Guidelines. Laboratory Support for the Diagnosis and Monitoring of Thyroid Disease. AACC Press, 2002.
- Ridker PM. High-sensitivity C-reactive protein and cardiovascular risk: rationale for screening and primary prevention. Am J Cardiol 2003;92:17K-22K. [Crossref] [PubMed]
- Apple FS. A new season for cardiac troponin assays: it's time to keep a scorecard. Clin Chem 2009;55:1303-6. [Crossref] [PubMed]
- Reichlin T, Schindler C, Drexler B, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med 2012;172:1211-8. [Crossref] [PubMed]
- Januzzi JL Jr, Mahler SA, Christenson RH, et al. Recommendations for Institutions Transitioning to High-Sensitivity Troponin Testing: JACC Scientific Expert Panel. J Am Coll Cardiol 2019;73:1059-77. [Crossref] [PubMed]
- Cook B, McCord J, Hudson M, et al. Baseline High Sensitivity Cardiac Troponin I Level Below Limit of Quantitation Rules Out Acute Myocardial Infarction in the Emergency Department. Crit Pathw Cardiol 2021;20:4-9. [Crossref] [PubMed]
- Boelstler AM, Rowland R, Theoret J, et al. Decreasing troponin turnaround time in the emergency department using the central laboratory: A process improvement study. Clin Biochem 2015;48:308-12. [Crossref] [PubMed]
- Wu AHB, Christenson RH, Greene DN, et al. Clinical Laboratory Practice Recommendations for the Use of Cardiac Troponin in Acute Coronary Syndrome: Expert Opinion from the Academy of the American Association for Clinical Chemistry and the Task Force on Clinical Applications of Cardiac Bio-Markers of the International Federation of Clinical Chemistry and Laboratory Medicine. Clin Chem 2018;64:645-55. [Crossref] [PubMed]
- Giannitsis E. Counterpoint: Potential Concerns Regarding the Use of Sex-Specific Cutpoints for High-Sensitivity Troponin Assays. Clin Chem 2017;63:264-6. [Crossref] [PubMed]
- Wu AH, Valdes R Jr, Apple FS, et al. Cardiac troponin-T immunoassay for diagnosis of acute myocardial infarction. Clin Chem 1994;40:900-7. [Crossref] [PubMed]
- International Federation of Clinical Chemistry Committee on Clinical Applications of Cardiac Bio-Marikers. High-sensitivity cardiac troponin I and T assay analytical characteristics designated by manufacturer. 2022. Available online: https://ifcc.web.insd.dk/media/479436/ifcc-cardiac-troponin-interference-table_v052022.pdf
- Cullen L, Collinson PO, Giannitsis E. Point-of-care testing with high-sensitivity cardiac troponin assays: the challenges and opportunities. Emerg Med J 2022;39:861-6. [Crossref] [PubMed]
- Wang M, Li J, Huang Y, et al. Analytical validation of the LiCA® high-sensitivity human thyroid stimulating hormone assay. Clin Biochem 2022;101:42-9. [Crossref] [PubMed]
- Vylegzhanina AV, Kogan AE, Katrukha IA, et al. Full-Size and Partially Truncated Cardiac Troponin Complexes in the Blood of Patients with Acute Myocardial Infarction. Clin Chem 2019;65:882-92. [Crossref] [PubMed]
Cite this article as: Wu AHB. A practical guide to the implementation of high-sensitivity cardiac troponin assays into the clinical lab. J Lab Precis Med 2023;8:10.


